大气羟基自由基路径的揭示:含矿物微滴气溶胶的界面介导效应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
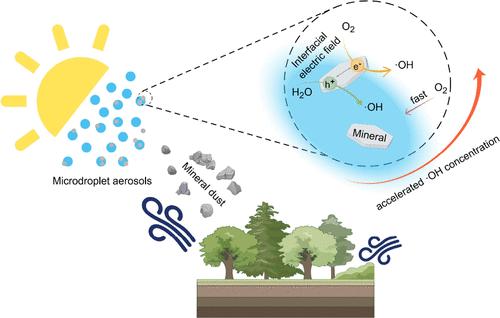
羟基自由基(·OH)在大气化学中起着至关重要的作用,调节氧化电位和气溶胶成分。这项研究揭示了大气中一个前所未有的·OH来源:含矿物粉尘的微滴气溶胶。我们证明了微滴气溶胶中的高岭土颗粒在太阳照射下触发快速的·OH生成,其速率至少达到10-3 M s-1的数量级。这种生产速度比体相(2.4 × 10-11 M s-1)和以前已知的途径高出几个数量级。在此基础上,估计在1 μm大小的气溶胶颗粒的气-水-固界面上,基于表面的界面·OH生成速率为8.9 × 10-5 mol m-2 s-1。·OH形成的增强归因于空气-水-固体界面的独特特征,其中由于在空气-水界面存在强电场,光诱导孔的寿命显着增加。我们进一步研究了各种环境因素和气溶胶特性对·OH产生的影响,包括光强、相对湿度、粒径和ph。我们的研究结果为含矿物粉尘微滴气溶胶介导的大气光化学过程提供了新的见解,这些气溶胶是大气中·OH源的重要贡献者。这项工作促进了我们对大气界面化学及其对空气质量和气候的深刻而持久的影响的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Atmospheric Hydroxyl Radical Route Revealed: Interface-Mediated Effects of Mineral-Bearing Microdroplet Aerosol
Hydroxyl radical (·OH) plays a crucial role in atmospheric chemistry, regulating the oxidative potential and aerosol composition. This study reveals an unprecedented source of ·OH in the atmosphere: mineral dust-bearing microdroplet aerosols. We demonstrate that Kaolin clay particles in microdroplet aerosols trigger rapid ·OH production upon solar irradiation, with rates reaching an order of at least 10–3 M s–1. This production rate is several orders of magnitude higher than that of the bulk phase (2.4 × 10–11 M s–1) and previously known pathways. On this basis, the surface-based interfacial ·OH production rate is estimated to be 8.9 × 10–5 mol m–2 s–1 at the air–water–solid interface of 1 μm sized aerosol particles. The enhanced ·OH formation is attributed to the unique features of air–water–solid interfaces, where the lifespan of photoinduced holes was significantly increased due to the presence of strong electric fields at the air–water interface. We further investigated the impacts of various environmental factors and aerosol properties on ·OH production, including light intensity, relative humidity, particle size, and pH. Our findings provide new insights into atmospheric photochemical processes mediated by mineral dust-bearing microdroplet aerosols, which are important contributors to ·OH source in the atmosphere. This work advances our understanding of atmospheric interfacial chemistry and its profound and lasting implications for air quality and climate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


