催化金属免疫治疗的自级联热分解- sting引发剂
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
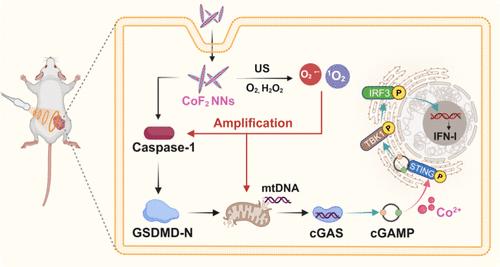
Gasdermin (GSDM)介导的焦亡涉及线粒体损伤的诱导和随后线粒体DNA (mtDNA)的释放,这有望激活cGAS-STING途径,从而增强抗肿瘤免疫反应。然而,挑战在于如何有效地触发癌细胞的焦亡,并随后特异性地增强cGAS-STING的激活。在此,我们开发了智能自级联热分解sting引发剂氟化钴(CoF2)纳米催化剂用于催化金属免疫治疗。具有半导体结构和酶样活性的CoF2纳米催化剂在内源H2O2和外源超声刺激下产生大量活性氧(ROS)。重要的是,我们发现钴基纳米材料本身可以诱导癌细胞的焦亡。因此,CoF2纳米催化剂最初作为焦亡诱导剂,通过Co2+和ROS触发癌细胞中caspase-1/ gsdmd依赖性焦亡,导致mtDNA释放。随后,CoF2纳米催化剂被进一步用作智能STING激动剂,能够特异性检测mtDNA并增强cGAS-STING通路的激活。这些级联事件触发了强大的免疫反应,有效地将免疫抑制的肿瘤微环境调节到免疫支持状态,从而为抗肿瘤治疗提供有利的支持。这一创新策略不仅显著阻碍了原发肿瘤的生长,而且还引发了免疫反应,进一步增强了免疫检查点抑制剂在阻止远处肿瘤进展方面的功效。总的来说,本研究提出了一种自级联策略,通过焦亡介导的特异性激活和扩增cGAS-STING通路,为未来的癌症催化金属免疫治疗提供了一条有价值的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Self-Cascaded Pyroptosis-STING Initiators for Catalytic Metalloimmunotherapy
Gasdermin (GSDM)-mediated pyroptosis involves the induction of mitochondrial damage and the subsequent release of mitochondrial DNA (mtDNA), which is anticipated to activate the cGAS-STING pathway, thereby augmenting the antitumor immune response. However, challenges lie in effectively triggering pyroptosis in cancer cells and subsequently enhancing the cGAS-STING activation with specificity. Herein, we developed intelligent self-cascaded pyroptosis-STING initiators of cobalt fluoride (CoF2) nanocatalysts for catalytic metalloimmunotherapy. CoF2 nanocatalysts with a semiconductor structure and enzyme-like activity generated a substantial amount of reactive oxygen species (ROS) under stimulation by endogenous H2O2 and exogenous ultrasound. Importantly, we discovered that Co-based nanomaterials themselves induce pyroptosis in cancer cells. Therefore, CoF2 nanocatalysts initially acted as pyroptosis inducers, triggering caspase-1/GSDMD-dependent pyroptosis in cancer cells via Co2+ and ROS, leading to mtDNA release. Subsequently, CoF2 nanocatalysts were further utilized as intelligent STING agonists that were specifically capable of detecting mtDNA and augmenting the activation of the cGAS-STING pathway. These cascade events triggered a robust immune response, effectively modulating the immunosuppressive tumor microenvironment into an immune-supportive state, thereby providing favorable support for antitumor therapy. This innovative strategy not only significantly impeded the growth of the primary tumor but also elicited an immune response to further augment the efficacy of immune checkpoint inhibitors in preventing distant tumor progression. Overall, this study proposed a self-cascade strategy for activating and amplifying the cGAS-STING pathway with specificity mediated by pyroptosis, representing a valuable avenue for future cancer catalytic metalloimmunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


