通过酶催化制备高烯化醛,用于β-羟基膦酸盐的对映互补构建
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
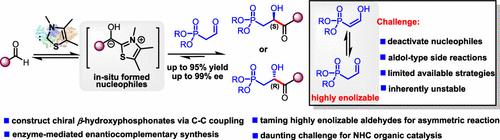
与广泛探索的简单烷基或芳基醛相比,驯服高烯化醛催化与亲核试剂的不对称C-C偶联仍然是一个难以捉摸的挑战。本文中,我们使用thdp依赖性酶实现了高度烯化的2-膦酸酯醛与原位生成的动态可逆亲核试剂(酰基阴离子)的直接C-C偶联。与nhc介导的产生多种加合物的复杂混合物的反应不同,我们的酶催化过程选择性地产生具有生物活性的β-羟基膦酸盐,产率高(高达95%),对端选择性好(高达99% ee)。产物可以在克尺度上获得,并表现出丰富的下游转化反应性,以提供不同的分子。PfBAL(或其突变体A28G)和PaBAL酶作为对映体互补对,能够合成两种产物构型。机理研究证明,这两种酶对的活性腔入口方向不同,导致这两种酶对形成的酰基阴离子从不同的方向攻击2-膦酸酯醛。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Taming Highly Enolizable Aldehydes via Enzyme Catalysis for Enantiocomplementary Construction of β-Hydroxyphosphonates
Taming highly enolizable aldehydes for catalytic asymmetric C–C coupling with nucleophiles remains an elusive challenge compared to widely explored simple alkyl or aryl aldehydes. Herein, we use ThDP-dependent enzymes to realize the direct C–C coupling of highly enolizable 2-phosphonate aldehydes with in situ-generated dynamically reversible nucleophiles (acyl anions). Unlike NHC-mediated reactions that yield complex mixtures of multiple adducts, our enzymatic process selectively produces biologically active β-hydroxy phosphonates with high yields (up to 95%) and excellent enantioselectivities (up to 99% ee). The products can be obtained on gram scales and exhibit rich reactivity for downstream transformations to afford diverse molecules. PfBAL (or its mutant A28G) and PaBAL enzymes serve as enantiocomplementary pairs, enabling the synthesis of both product configurations. Mechanistic studies proved that the entrance directions of the active cavities of these two enzyme pairs were distinct, leading to acyl anions formed from these two enzyme pairs attacking 2-phosphonate aldehydes from different orientations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


