在De Novo设计的肽桶内的约束和催化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
从头开始的蛋白质设计已经取得了进步,许多肽组装和蛋白质结构可以预测和快速地产生。现在的动力是给这些结构带来功能,比如小分子结合和催化。结合和定向多个小分子以指导化学的艰巨挑战对于为新功能铺平道路尤为重要。为了解决这个问题,我们在这里描述了水溶液中小分子肽三元配合物的设计、表征和应用。这使用α-螺旋桶(α hb)肽组件,它由5个或更多的α螺旋组成,排列在中心通道周围。这些通道是溶剂可接近的,它们的内部尺寸和化学性质可以预测地改变。因此,αHBs类似于在超分子、聚合物和材料化学中制造的“分子烧瓶”。利用Förster共振能量转移作为读数,我们证明了特定的αHBs可以近距离地接受两种不同的有机染料,1,6-二苯基-1,3,5-己三烯和尼罗河红。此外,两个蒽分子可以在αHB内容纳,以促进蒽的光二聚化。然而,并不是所有的三元配合物都是有效的,无论是在能量转移或光二聚化,说明可以通过明智的选择和αHB的设计发挥控制作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
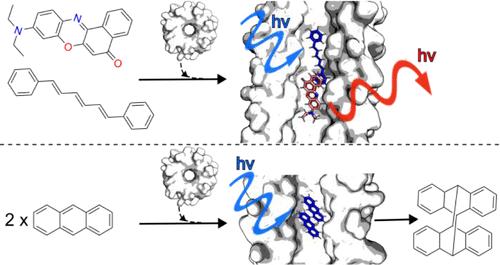
Confinement and Catalysis within De Novo Designed Peptide Barrels
De novo protein design has advanced such that many peptide assemblies and protein structures can be generated predictably and quickly. The drive now is to bring functions to these structures, for example, small-molecule binding and catalysis. The formidable challenge of binding and orienting multiple small molecules to direct chemistry is particularly important for paving the way to new functionalities. To address this, here we describe the design, characterization, and application of small-molecule:peptide ternary complexes in aqueous solution. This uses α-helical barrel (αHB) peptide assemblies, which comprise 5 or more α helices arranged around central channels. These channels are solvent accessible, and their internal dimensions and chemistries can be altered predictably. Thus, αHBs are analogous to “molecular flasks” made in supramolecular, polymer, and materials chemistry. Using Förster resonance energy transfer as a readout, we demonstrate that specific αHBs can accept two different organic dyes, 1,6-diphenyl-1,3,5-hexatriene and Nile red, in close proximity. In addition, two anthracene molecules can be accommodated within an αHB to promote anthracene photodimerization. However, not all ternary complexes are productive, either in energy transfer or photodimerization, illustrating the control that can be exerted by judicious choice and design of the αHB.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


