不同的C(sp3) -H通过电化学苯氧化功能化
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-01-22
DOI:10.1039/d4qo02022g
引用次数: 0
摘要
尽管在C(sp3) -H键功能化方面取得了重大进展,但开发具有位点选择性和能够形成多种键的方法仍然是一个挑战。此外,由于缺乏立体选择性和过度氧化,通过裂解相邻的C(sp3) -H键从脂肪族制备烯烃仍然很困难。为了应对这些挑战,我们开发了一种无过渡金属催化剂和外部无氧化剂的电化学C(sp3) - h氧化平台,该平台能够高度立体选择性地合成芳基烯烃和各种苯基功能化,形成各种C - C和C -杂原子键。这种方法包括位点选择性的苯基C(sp3) - h三氟乙酰氧基化,然后用不同的碳基或杂原子基亲核试剂消除或取代,形成功能化产物。脱氢枞酸甲酯在十元尺度上的脱饱和和生成的烯烃的转化进一步证明了该方法的实用性。本研究为简单烷基芳烃和复杂分子的多样性功能化提供了一个平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。

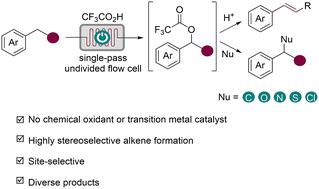
Diverse C(sp3)–H functionalizations through electrochemical benzylic oxygenation†
Despite significant advances in C(sp3)–H bond functionalization, the development of methods that are site-selective and capable of diverse bond formation remains a challenge. In addition, the preparation of olefins from aliphatics through cleavage of vicinal C(sp3)–H bonds remains difficult due to a lack of stereoselectivity and over-oxidation. To address these challenges, we have developed a transition metal catalyst- and external oxidant-free electrochemical C(sp3)–H oxygenation platform that enables highly stereoselective synthesis of aryl alkenes and diverse benzylic functionalizations to form various C–C and C–heteroatom bonds. This method involves site-selective benzylic C(sp3)–H trifluoroacetoxylation followed by elimination or substitution with diverse carbon- or heteroatom-based nucleophiles to form functionalized products. The utility of this protocol is further demonstrated by the desaturation of methyl dehydroabietate at the decagram scale and transformations of the resulting alkene. This study provides a platform for the diversity-oriented functionalization of both simple alkyl arenes and complex molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


