纯Pt及Pt基双金属合金(111)单晶表面吸附硫的电化学氧化脱附
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
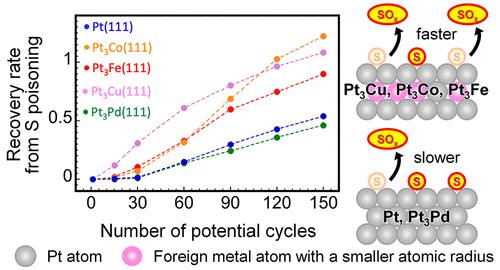
采用电化学测量和x射线光电子能谱(XPS)研究了纯Pt和各种Pt基双金属合金Pt3M (M = Co, Cu, Fe, Pd)(111)表面硫的吸附/解吸行为。单质硫吸附后,无硫裸(111)表面氢和羟基吸附/解吸的电流响应特征完全消失,在XPS光谱S 2p区出现单质硫对应的双峰。通过在- 0.2 ~ 0.8 V V /Ag /AgCl之间重复电位循环,特征电流响应逐渐恢复,同时S 2p峰降低,表明S种氧化脱附发生。除了Pt3Pd(111)表面Pd具有与Pt相似的原子半径和完全占据的4d轨道外,Pt3M(111)表面表现出比纯Pt(111)表面更高的氧化脱附能力;与Pt(111)表面相比,Pt3M(111)表面通过更少的电位循环恢复了电化学活性表面积。其中,外来金属的配体效应导致的d带中心的下移以及吸附的S与Pt之间的电子相互作用是促进硫氧化解吸以及原子半径小于Pt的外来金属的应变效应的主要因素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electrochemical Oxidative Desorption of Adsorbed Sulfur Species on (111) Surfaces of Single Crystals of Pure Pt and Pt-Based Bimetallic Alloys
The adsorption/desorption behavior of sulfur species at the (111) surfaces of pure Pt and various Pt-based bimetallic alloys, denoted as Pt3M (M = Co, Cu, Fe, Pd), was investigated by electrochemical measurements and X-ray photoelectron spectroscopy (XPS). After the adsorption of elemental sulfur, the current responses characteristic of the adsorption/desorption of hydrogen and hydroxyl species at the sulfur-free bare (111) surfaces completely disappeared, and a doublet peak corresponding to the elemental sulfur appeared in the S 2p region of XPS spectra. The characteristic current responses gradually recovered, simultaneously with the decrease of the S 2p peak, by repeating the potential cycling between −0.2 and 0.8 V vs Ag/AgCl, indicating the oxidative desorption of S species. Except for the Pt3Pd(111) surface, in which Pd has a similar atomic radius to Pt and fully occupied 4d orbitals, the Pt3M(111) surfaces showed higher oxidative desorption capability than those of the pure Pt(111) surface; electrochemically active surface area recovered at the Pt3M(111) surfaces by fewer potential cycles than at the Pt(111) surface. Among the various factors, the downshift of the d-band center due to the ligand effect of foreign metal and the electronic interaction between adsorbed S and Pt are the dominant factors promoting the oxidative desorption of sulfur as well as the strain effect of foreign metal with an atomic radius smaller than Pt.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


