氢分子在氢化钙低温氧化还原中的还原剂作用
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
低温合成对于推进可持续制造和获得新的亚稳相至关重要。金属氢化物在促进氧化物的低温还原方面显示出巨大的潜力,但其潜在的机制──是由H -、H2还是H原子驱动──仍不清楚。在这项研究中,我们采用原位电输运测量和第一性原理计算来研究cah2驱动的α-Fe2O3外延薄膜的还原动力学。有趣的是,与CaH2粉末直接接触或分离的样品表现出相似的H2还原表观活化能,尽管直接接触显著提高了还原速率。这些发现表明,H2分子是CaH2低温还原氧化物的主要还原物质,氢化物具有优异的还原能力的一个关键方面是它们能够消除残余水分。这项工作强调了水分控制在实现先进材料合成的有效低温氧化物还原中的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
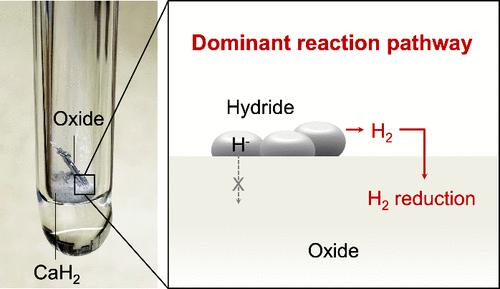
Molecular H2 as the Reducing Agent in Low-Temperature Oxide Reduction Using Calcium Hydride
Low-temperature synthesis is crucial for advancing sustainable manufacturing and accessing novel metastable phases. Metal hydrides have shown great potential in facilitating the reduction of oxides at low temperatures, yet the underlying mechanism─whether driven by H–, H2, or atomic H─remains unclear. In this study, we employ in situ electrical transport measurements and first-principles calculations to investigate the CaH2-driven reduction kinetics in epitaxial α-Fe2O3 thin films. Intriguingly, samples in direct contact with or separated from CaH2 powders exhibit similar apparent activation energies for H2 reduction, although direct contact significantly increases the reduction rate. These findings indicate that molecular H2 is the dominant reducing species in the low-temperature reduction of oxides using CaH2, with a key aspect of the hydrides’ superior reducing power attributed to their ability to eliminate residual moisture. This work underscores the critical role of moisture control in enabling effective low-temperature oxide reduction for advanced material synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


