氚在Li8PbO6中的安置和扩散的第一性原理模拟
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
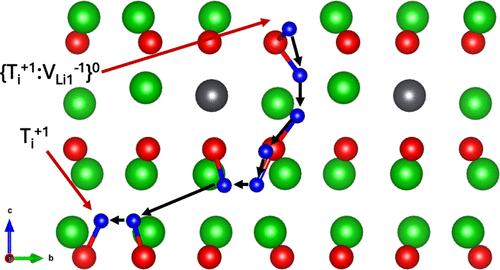
Li8PbO6已被提出作为一种备选的用于聚变反应堆的增殖包层材料。当锂在毯内燃烧时,氚在陶瓷基体中产生,直到它到达表面,从那里通过同位素交换反应回收。为了充分了解氚的回收过程,有必要了解氚是如何被安置在燃料中并随后迁移到表面的。因此,在这项工作中,我们采用密度泛函理论(DFT)来研究氚在Li8PbO6中的安置。然后,我们使用微推弹性带(NEB)方法来了解Li8PbO6中容纳氚缺陷的迁移机制。我们发现氚更可能与氧离子结合并形成羟基,而不是存在于传统的间隙中。我们预测氚间隙的迁移势垒是各向异性的,沿x平面和z轴的势垒分别为0.27和0.69 eV。锂空位捕获点的逃逸势垒在0.76 ~ 0.85 eV之间,整个捕获点的迁移活化能在0.67 ~ 1.18 eV之间。由于发现的迁移能较低,我们预测与其他主要候选材料相比,毯层的老化对氚释放的影响较小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tritium Accommodation and Diffusion in Li8PbO6 from First-Principles Simulations
Li8PbO6 has been proposed as an alternative candidate breeding blanket material for use in fusion reactors. As lithium is burned inside the blanket, tritium is produced within the ceramic matrix until it reaches the surface, from where it is recovered by isotope exchange reactions. To fully understand the tritium recovery process, it is essential to understand how tritium is accommodated in the fuel and subsequently migrates to the surface. Therefore, in this work, we employ density functional theory (DFT) to examine tritium accommodation in Li8PbO6. We then used the nudged elastic band (NEB) method to understand the mechanisms for the migration of tritium-accommodating defects in Li8PbO6. We have found tritium is more likely bind to an oxygen ion and form a hydroxyl than exist in the traditional interstitial sites. We predict the barriers for migration of tritium interstitials to be anisotropic, with barriers of 0.27 and 0.69 eV along the xy-plane and through the z-axis, respectively. The barrier for escape from a lithium vacancy trapping site we found to be in the range of 0.76–0.85 eV, and an activation energy range of 0.67–1.18 eV for the migration of the trapping site as a whole. Due to the low migration energies found, we predict that aging of the blanket will have a lower significance on tritium release compared to other leading candidate materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


