一线化疗免疫治疗SCLC:两国真实世界研究的结果。
IF 3.5
Q2 ONCOLOGY
引用次数: 0
摘要
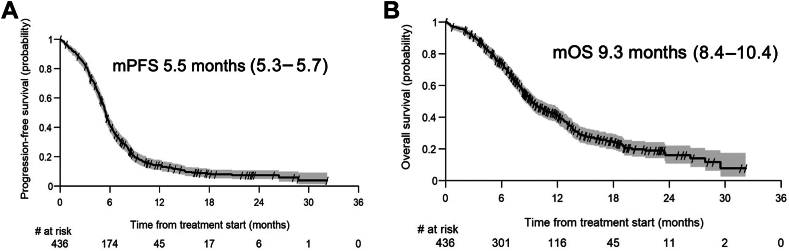
SCLC的特点是侵袭性和有限的治疗选择,特别是大分期SCLC (ES-SCLC)。在这种情况下,在铂-依托泊苷联合治疗的基础上增加免疫治疗最近已成为标准。这项回顾性研究旨在评估化学免疫治疗对ES-SCLC患者的实际疗效,重点关注临床试验排除的亚群。方法:一项回顾性的两国多中心研究,包括来自10个英国和10个瑞士机构的ES-SCLC患者。患者接受铂-依托泊苷化疗联合免疫治疗(atezolizumab或durvalumab)。收集患者、肿瘤和治疗细节。分析了总生存期(OS)、无进展生存期、客观缓解率和安全性结果。结果:共纳入436例患者。在我们的队列中,142名患者(32.6%)由于东部肿瘤合作组的表现状态为2或更高、自身免疫性疾病、活动性脑转移或类固醇使用而不符合关键注册试验的资格。大多数患者接受卡铂(96.8%)和atezolizumab(97.9%)。中位无进展生存期为5.5个月,中位OS为9.3个月。两年生存率为14%。肝或骨转移或东部肿瘤合作组表现为2或更高的患者生存结果较差。222名患者(51%)报告了与治疗相关的不良事件,而95名患者(22%)报告了与免疫相关的不良事件。5级免疫相关不良事件中有3 / 5是由肺炎引起的。结论:据我们所知,这是现实世界中接受ES-SCLC化疗免疫治疗的最大患者队列。尽管三分之一的患者不符合关键试验的条件,但我们队列中的生存结果与注册试验相似。特别是,长期幸存者的数量和安全性数据具有可比性,支持在日常临床实践中使用化学免疫疗法作为ES-SCLC的一线治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
First-Line Chemo-Immunotherapy in SCLC: Outcomes of a Binational Real-World Study
Introduction
SCLC is characterized by aggressiveness and limited treatment options, especially in extensive-stage SCLC (ES-SCLC). Immunotherapy added to the platinum-etoposide combination has recently become standard in this setting. This retrospective study aims to evaluate the real-world effectiveness of chemo-immunotherapy in patients with ES-SCLC, focusing on subpopulations excluded from clinical trials.
Methods
A retrospective binational multicenter study was conducted, involving consecutive patients with ES-SCLC from 10 British and 10 Swiss institutions. Patients received platinum-etoposide chemotherapy in combination with immunotherapy (atezolizumab or durvalumab). Patient, tumor, and treatment details were collected. Overall survival (OS), progression-free survival, objective response rate, and safety outcomes were analyzed.
Results
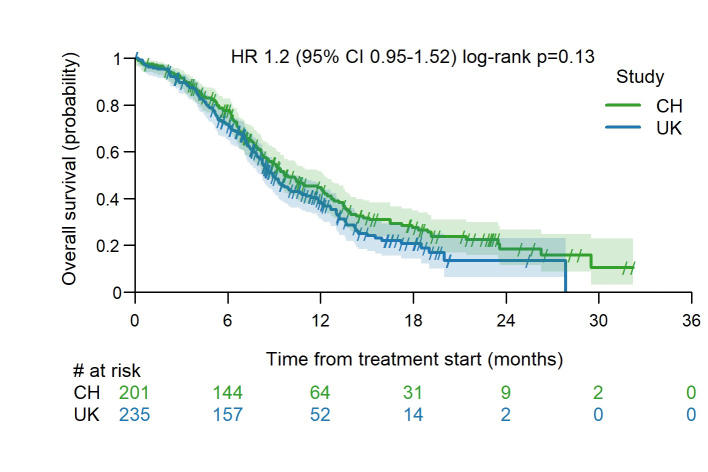
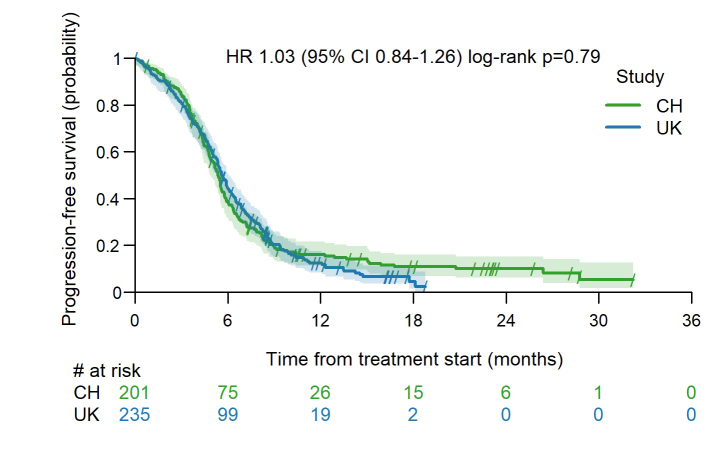
A total of 436 patients were included. One hundred forty-two patients (32.6%) in our cohort would not have been eligible for the pivotal registrational trials owing to an Eastern Cooperative Oncology Group performance status of 2 or higher, autoimmune disease, active brain metastases, or steroid use. Most patients received carboplatin (96.8%) and atezolizumab (97.9%). The median progression-free survival was 5.5 months and the median OS was 9.3 months. The two-year OS was 14%. Patients with liver or bone metastases or an Eastern Cooperative Oncology Group performance status of 2 or higher had worse survival outcomes. Treatment-related adverse events were reported in 222 patients (51%) whereas immune-related adverse events occurred in 95 patients (22%). Three out of five grade 5 immune-related adverse events were caused by pneumonitis.
Conclusions
To our knowledge, this is the largest real-world cohort of patients treated with chemo-immunotherapy for ES-SCLC. Although one-third of patients would not have been eligible for pivotal trials, the survival outcomes in our cohort are similar to those in registrational trials. In particular, the number of long-term survivors and the safety data are comparable, supporting the use of chemo-immunotherapy as first-line treatment for ES-SCLC in daily clinical practice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JTO Clinical and Research Reports
Medicine-Oncology
CiteScore
4.20
自引率
0.00%
发文量
145
审稿时长
19 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


