WT1疫苗(Galinpepimut-S)与检查点抑制(Nivolumab)联合治疗表达WT1的弥漫胸膜间皮瘤患者:一项1期研究
IF 3.5
Q2 ONCOLOGY
引用次数: 0
摘要
简介:WT1常出现在弥漫性胸膜间皮瘤(DPMs)表面,是理想的治疗靶点。Galinpepimut-S(GPS)是一种四价、非人类白细胞抗原限制性、异体WT1特异性多肽疫苗,在早期临床试验中安全有效,并能在其他恶性肿瘤的肿瘤微环境中上调T细胞抑制性程序性死亡配体1。一项关于DPM患者辅助GPS的随机2期研究显示,中位总生存期有改善趋势:为了进一步提高免疫原性,我们在一项开放标签、单中心的1期研究中将GPS与抗PD1单克隆抗体nivolumab联合使用,考察了既往接受过治疗的DPM患者的耐受性和免疫原性。我们招募了至少接受过一个疗程培美曲塞化疗的进展期或复发性DPM患者。患者先接受2个剂量的GPS治疗,然后接受6个剂量的GPS治疗,每2周静脉注射一次nivolumab,最多可再接受6个周期的治疗,直至疾病进展或出现不可接受的毒性反应:10名患者接受了治疗;70%的患者出现了轻微的治疗相关不良反应;2名患者出现了3级或以上不良反应。10名患者中有3名(30%)报告了疫苗特异性T细胞应答。没有部分应答;3名患者病情稳定时间延长,肿瘤体积减少达17%。中位无进展生存期为3.9个月,中位总生存期为7.4个月:结论:GPS和nivolumab联合用药具有可耐受的毒性,可诱导部分患者产生免疫应答,但可能由于样本量较小,初始应答和生存获益有限。本文章由计算机程序翻译,如有差异,请以英文原文为准。
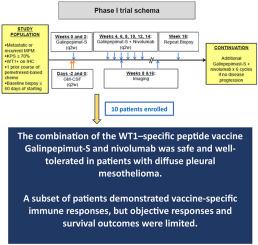
Combining a WT1 Vaccine (Galinpepimut-S) With Checkpoint Inhibition (Nivolumab) in Patients With WT1–Expressing Diffuse Pleural Mesothelioma: A Phase 1 Study
Introduction
WT1 often presents on the surface of diffuse pleural mesotheliomas (DPMs) and is an ideal therapeutic target. Galinpepimut-S (GPS), a tetravalent, non–human leukocyte antigen–restricted, heteroclitic WT1–specific peptide vaccine was safe and effective in early phase clinical trials and upregulates T-cell suppressive programmed death-ligand 1 in the tumor microenvironment of other malignancies. A randomized phase 2 study of adjuvant GPS in patients with DPM trended toward improved median overall survival.
Methods
To further enhance immunogenicity, we combined GPS with nivolumab, an anti-PD1 monoclonal antibody, in an open-label, single-center phase 1 study, examining tolerability and immunogenicity in patients with previously treated DPM. We enrolled patients with progressive or recurrent DPM treated with at least one course of pemetrexed-based chemotherapy. Patients received two doses of GPS followed by six doses of GPS with intravenous nivolumab every 2 weeks, and up to six additional cycles until disease progression or unacceptable toxicity.
Results
Ten patients were treated; 70% experienced mostly mild treatment-related adverse events; two experienced a grade 3 or higher adverse event. Three of the 10 patients (30%) reported vaccine-specific T-cell responses. There were no partial responses; three patients had prolonged stable disease with up to 17% decrease in tumor volume. Median progression-free survival was 3.9 months and the median overall survival was 7.4 months.
Conclusions
Coadministration of GPS and nivolumab reported a tolerable toxicity profile and induced immune responses in a subset of patients, but initial response and survival benefit were limited possibly owing to the small sample size.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JTO Clinical and Research Reports
Medicine-Oncology
CiteScore
4.20
自引率
0.00%
发文量
145
审稿时长
19 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


