适合目的测试和独立的GMP验证单核细胞活化试验。
IF 2.9
Q2 TOXICOLOGY
引用次数: 0
摘要
本研究描述了用于检测热原和促炎污染物的单核细胞活化试验(MAT)的“适合目的”测试和独立产品特异性GMP验证,MAT方法A,定量测试(欧洲药典,Ph. Eur)。来自2.6.30章,2017)。进行了一项适合目的的研究,以确保所选择的MAT装置(冷冻保存的PBMC, IL-6检测)能够可靠地区分含有低于污染物极限浓度(CLC)的热原污染物批次和含有高于CLC的热原污染物批次。这种测试在选定的MAT设备用于后续产品测试之前进行一次,以显示假阳性(热原阴性(CLC)批次)和假阴性(热原阳性(>CLC))测试的发生率为热原阴性(本文章由计算机程序翻译,如有差异,请以英文原文为准。
Fit for purpose testing and independent GMP validation of the monocyte activation test
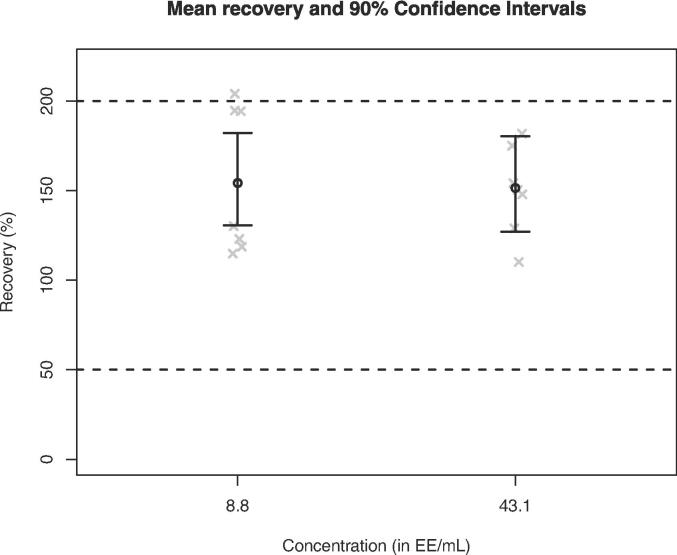
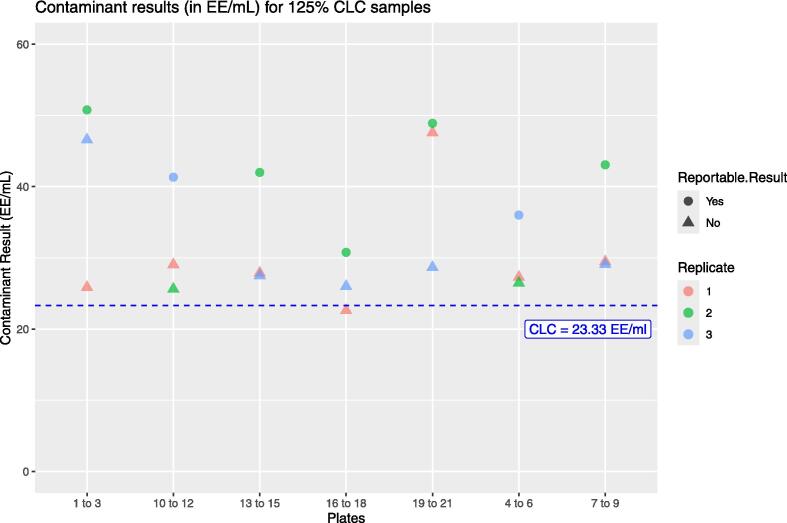
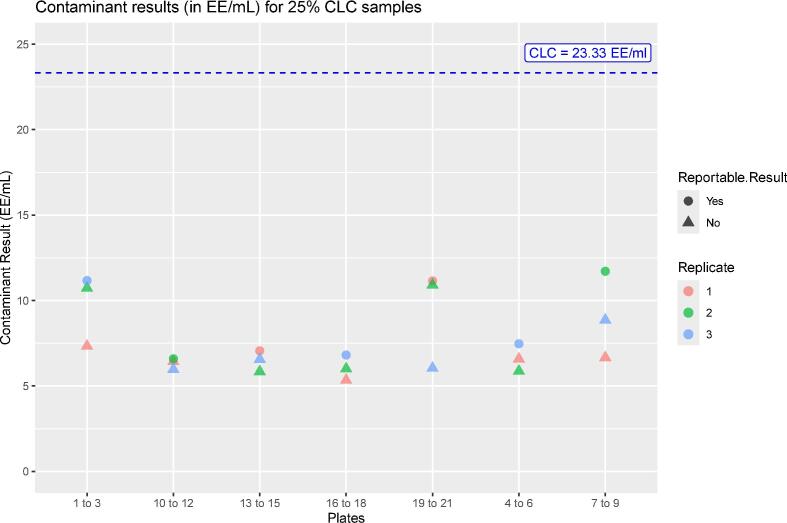
The present study describes the “fit for purpose” testing and the independent product-specific GMP validation of the monocyte activation test (MAT) to detect pyrogenic and pro-inflammatory contaminants, MAT Method A, Quantitative Test (European Pharmacopoeia, Ph. Eur. chapter 2.6.30, 2017). A fit for purpose study was carried out to ensure that the chosen MAT set-up (cryopreserved PBMC, IL-6 detection) can reliably discriminate between batches of product containing pyrogenic contaminants below the contaminants limit concentration, CLC, from batches containing pyrogenic contaminants above the CLC. Such testing is carried out once, before the chosen MAT set-up is used for subsequent product testing to show that the incidence of false positives (pyrogen-negative (<CLC) batches testing as pyrogen-positive (>CLC) batches) and – especially – false negatives (pyrogen-positive (>CLC) testing as pyrogen-negative (<CLC)) is low. This study also afforded the opportunity to collect an independent body of validation data for comparison with that obtained previously (Daniels et al., 2022) to evaluate the robustness of MAT Method A and its fitness to replace the rabbit pyrogen test (RPT) where this has not already happened.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Toxicology
Environmental Science-Health, Toxicology and Mutagenesis
CiteScore
4.70
自引率
3.00%
发文量
33
审稿时长
82 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


