用路易斯对在单原子尺度上鉴定碱水电解的双功能机理
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
双功能机制,包括多活性成分同时解离水分子和优化中间吸附,已广泛用于催化剂的设计,以促进水电解可持续氢能源生产,但由于难以准确识别反应过程仍存在争议。在此,我们提出了具有独特酸碱性质的单原子催化剂中明确定义的Lewis对的概念,以全面了解多活性成分在碱性析氢反应中的确切作用。通过调节活性基团,诱导Lewis对(M - p /S/Cr对,M = Ru, Ir, Pt)之间的协同效应可以显著促进H-OH键的断裂,加速中间产物的去除,从而将速率决定步骤从Volmer步骤转换为Heyrovsky步骤。此外,具有代表性的Ru-P Lewis对在2 a cm-2的高工业电流密度下提供了令人印象深刻的266 h耐久性,在阴离子交换膜电解中没有活性衰减,并且该概念可以扩展到修改商业贵金属基催化剂以增强性能。这项工作不仅揭示了双功能机理在单原子尺度碱性水电解中的重要作用,而且为先进催化剂的合理设计提供了一个通用描述符。本文章由计算机程序翻译,如有差异,请以英文原文为准。
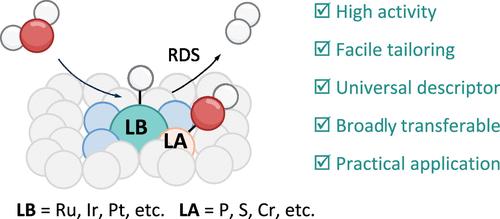
Identifying the Bifunctional Mechanism in Alkaline Water Electrolysis by Lewis Pairs at the Single-Atom Scale
The bifunctional mechanism, involving multiactive compositions to simultaneously dissociate water molecules and optimize intermediate adsorption, has been widely used in the design of catalysts to boost water electrolysis for sustainable hydrogen energy production but remains debatable due to difficulties in accurately identifying the reaction process. Here, we proposed the concept of well-defined Lewis pairs in single-atom catalysts, with a unique acid–base nature, to comprehensively understand the exact role of multiactive compositions in an alkaline hydrogen evolution reaction. By facilely adjusting active moieties, the induced synergistic effect between Lewis pairs (M–P/S/Cr pairs, M = Ru, Ir, Pt) can significantly facilitate the cleavage of the H–OH bond and accelerate the removal of intermediates, thereby switching the rate-determining step from the Volmer step to the Heyrovsky step. Moreover, the representative Ru–P Lewis pairs deliver an impressive 266 h durability at a high industrial current density of 2 A cm–2 without activity decay in anion-exchange membrane water electrolysis, and the concept can be extended to modify commercial noble-metal-based catalysts for performance enhancement. This work not only sheds light on the important effect of the bifunctional mechanism in alkaline water electrolysis at the single-atom scale but also offers a universal descriptor for the rational design of advanced catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


