酶自固定标记和生物正交反应的预靶向多模式肿瘤成像
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
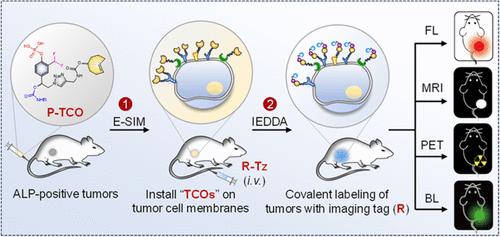
细胞膜共价修饰已显示出肿瘤成像和治疗的希望。然而,现有的膜标记技术面临着动力学慢、对癌细胞选择性差等挑战,导致脱靶效应和体内效果不理想。在这里,我们提出了一种酶触发的自固定标记策略,称为E-SIM,它可以在体内用生物正交反式环切烯(TCO)处理快速和选择性地标记肿瘤细胞膜。E-SIM利用P-TCO,一种碱性磷酸酶(ALP)响应的醌甲基(QM)前体与TCO基团,通过接近标记促进高密度TCO把手快速结合到肿瘤细胞膜上。然后,这些TCO基团通过快速生物正交反应与含四氮(Tz)的报告蛋白有效反应,导致肿瘤细胞膜上各种大小和成像方式的报告蛋白显著富集。我们证明了E-SIM标记和生物正交反应在体内肿瘤预靶向多模成像中的有效性。值得注意的是,我们在体内实现了选择性、高效地将z修饰的Renilla荧光素酶安装在肿瘤细胞上,从而为检测和指导人类HepG2肝肿瘤腹膜转移的手术切除提供了高度敏感的生物发光信号。E-SIM代表了在复杂的体内环境中精确标记肿瘤细胞的强大工具,可用于肿瘤中多模态成像应用的各种报告基因的预靶向富集。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Pretargeted Multimodal Tumor Imaging by Enzymatic Self-Immobilization Labeling and Bioorthogonal Reaction
Covalent modification of cell membranes has shown promise for tumor imaging and therapy. However, existing membrane labeling techniques face challenges such as slow kinetics and poor selectivity for cancer cells, leading to off-target effects and suboptimal in vivo efficacy. Here, we present an enzyme-triggered self-immobilization labeling strategy, termed E-SIM, which enables rapid and selective labeling of tumor cell membranes with bioorthogonal trans-cycloctene (TCO) handles in vivo. E-SIM utilizes P-TCO, an alkaline phosphatase (ALP) responsive quinone methide (QM) precursor with a TCO group, facilitating the rapid conjugation of high-density TCO handles onto tumor cell membranes via proximity labeling. These TCO groups then react efficiently with tetrazine (Tz)-bearing reporters via a fast bioorthogonal reaction, resulting in significant enrichment of reporters of various sizes and imaging modalities on tumor cell membranes. We demonstrate the efficacy of E-SIM labeling and bioorthogonal reaction for pretargeted multimodality imaging of tumors in vivo. Notably, we achieve selective and efficient installation of Tz-modified Renilla luciferase on tumor cells in vivo, thereby offering highly sensitive bioluminescence signals for detecting and guiding the surgical removal of small human HepG2 liver tumor peritoneal metastases. E-SIM represents a robust tool for precise tumor cell labeling in complex in vivo environments, feasible for pretargeted enrichment of various reporters in tumors for multimodal imaging applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


