手性醛/钯催化实现了亚甲基环丙烷与氨基酸酯的不对称支链选择性开环功能化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
由于涉及复杂的反应伙伴,通过选择性C-C键裂解实现亚甲基环丙烷(MCPs)的催化不对称功能化是一个显着的挑战。在这项工作中,我们报道了手性醛/钯联合催化使MCPs与nh2无保护的氨基酸酯不对称功能化。该反应通过区域特异性的支链开环机制进行,产生具有光学活性的α,α-二取代α-氨基酸酯,其末端具有非共轭烯烃单元。机理研究表明,开环途径是不可逆的,最终的区域选择性由钯的催化作用决定。该产物可用于构建手性二氢吡唑、α-甲基天冬氨酸衍生物以及VPC01091和BMS-986104的类似物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
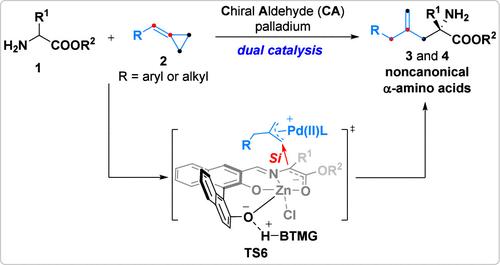
Chiral Aldehyde/Palladium Catalysis Enables Asymmetric Branched-Selective Ring-Opening Functionalization of Methylenecyclopropanes with Amino Acid Esters
Achieving catalytic asymmetric functionalization of methylenecyclopropanes (MCPs) by selective C–C bond cleavage is a notable challenge due to the intricate reaction partners involved. In this work, we report that chiral aldehyde/palladium combined catalysis enables the asymmetric functionalization of MCPs with NH2-unprotected amino acid esters. This reaction proceeds through a regiospecific branched ring-opening mechanism, resulting in optically active α,α-disubstituted α-amino acid esters bearing nonconjugated terminal alkene units. Mechanism studies indicate that the ring-opening pathways are irreversible and the ultimate regioselectivity is governed by palladium catalysis. The products can be utilized in the construction of chiral dihydropyrazoles, α-methyl aspartic acid derivatives, and analogues of VPC01091 and BMS-986104.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


