叠氮嘧啶的动态动力学活化使其与1,3-二烯的自由基-极性交叉(4 + 3)环加成成为可能
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
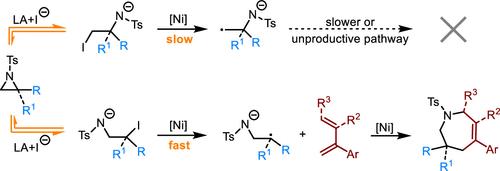
氮嘧啶与不饱和化合物的环加成是合成氮杂环的一种有价值的方法。然而,这一过程主要是由底物控制的,在调节C-N键切割的区域选择性方面提出了重大挑战。在这项研究中,我们报道了一种镍催化的动态动力学活化策略,使催化剂控制的氮杂环的活化成为可能。各种类型的叠氮嘧啶,包括2-苯基、2-羰基、2-烷基和二取代叠氮嘧啶,都能不断地劈开它们更具空间阻碍的C-N键,生成1,3自由基阴离子中间体。这些中间体与芳香支链1,3-二烯参与高度区域选择性的1,4- heck /烯丙基取代级联反应,导致自由基-极性交叉(4 + 3)环加成,产生七元氮卓类产物。这种方法不仅补充了传统的偶极环加成,在传统的偶极环加成中,氮嘧啶通常作为两性离子1,3偶极子,而且还为1,3-二烯引入了一种不同寻常的环加成模式。实验研究和密度泛函理论(DFT)计算提供了对反应机理的深入了解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dynamic Kinetic Activation of Aziridines Enables Radical-Polar Crossover (4 + 3) Cycloaddition with 1,3-Dienes
The cycloaddition of aziridines with unsaturated compounds is a valuable method for synthesizing nitrogen heterocycles. However, this process is predominantly substrate-controlled, posing significant challenges in regulating the regioselectivity of the C–N bond cleavage. In this study, we report a nickel-catalyzed dynamic kinetic activation strategy that enables catalyst-controlled activation of aziridines. Various types of aziridines, including 2-phenyl, 2-carbonyl, 2-alkyl, and disubstituted aziridines, consistently cleave their more sterically hindered C–N bonds to generate 1,3-radical anion intermediates. These intermediates participate in a highly regioselective 1,4-Heck/allylic substitution cascade with aromatic branched 1,3-dienes, resulting in a radical-polar crossover (4 + 3) cycloaddition that produces seven-membered azepine products. This approach not only complements traditional dipolar cycloaddition, in which aziridines typically act as zwitterionic 1,3-dipoles, but also introduces an unusual cycloaddition mode for 1,3-dienes. Experimental investigations and density functional theory (DFT) calculations provide insight into the reaction mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


