未活化亚胺的Pd-TMM环化引发的一锅多米诺催化构建烷基/芳基吡咯
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文描述了一锅多米诺骨牌催化的三组分工艺,该工艺由钯/锌协同催化的三甲基乙烷(TMM)和未活化的烷基/芳基亚胺之间的环加成引发,然后是一锅异构化和Zn(OTf)2催化的DDQ氧化,提供有价值的取代吡罗。我们发现钯/锌协同催化提供了一个双Zn(OTf)2稳定的氮杂环,其中Pd-N键被Zn(OTf)2极化,促进了独特的外球烯丙基胺化。此外,锌催化可以促进DDQ的后续脱氢。本文章由计算机程序翻译,如有差异,请以英文原文为准。
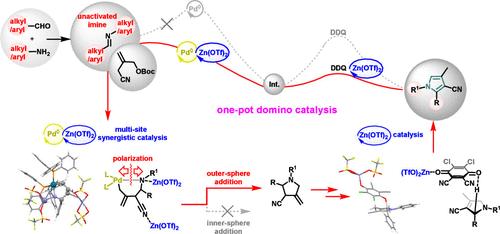
One-Pot Domino Catalysis to Construct Alkyl/Aryl Pyrroles Initiated by Pd-TMM Annulation of Unactivated Imines
Herein, a one-pot domino catalyzed three-component process is described, which is initiated by a palladium/zinc cooperatively catalyzed cycloaddition between trimethylenemethane (TMM) and unactivated alkyl/aryl imines, followed by one-pot isomerization and Zn(OTf)2-catalyzed DDQ oxidation, furnishing valuable substituted pyrroles. We disclose that the palladium/zinc cooperative catalysis affords a dual-Zn(OTf)2-stabilized azapalladacycle, wherein the Pd–N bond is polarized by Zn(OTf)2, facilitating a unique outer-sphere allylic amination. Moreover, subsequent DDQ dehydrogenation can be feasibly promoted by zinc catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


