三自由基分选使烯烃氨基烷基化
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
直接合成富含C(sp3)的结构是合成有机化学创新的推动力。这种支架赋予药物分子有益的特性,与更大的临床成功相关。因此,开发从商业原料(如烯烃)中获取富含sp3的分子的新方法具有强烈的推动力。在此,我们报道了一个三组分氨基烷基化反应,利用三自由基分选原理在烯烃上选择性地添加n中心和c中心自由基。该过程依赖于光氧化还原催化,以氧化还原中性的方式将烷基溴和还原活化的n中心自由基前体转化为高能自由基。广泛的偶联伙伴被证明具有多种合成应用,包括药效取代的n -杂环的简单合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
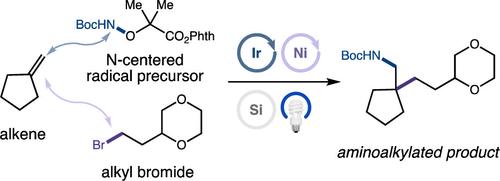
Aminoalkylation of Alkenes Enabled by Triple Radical Sorting
The direct synthesis of C(sp3)-rich architectures is a driving force for innovation in synthetic organic chemistry. Such scaffolds impart beneficial properties onto drug molecules that correlate with greater clinical success. Consequently, there is a strong impetus to develop new methods by which to access sp3-rich molecules from commercial feedstocks, such as alkenes. Herein, we report a three-component aminoalkylation reaction that utilizes the principles of triple radical sorting to regioselectively add N-centered and C-centered radicals across alkenes. This process relies upon photoredox catalysis to transform alkyl bromides and reductively activated N-centered radical precursors into high-energy radical species in a redox-neutral fashion. A broad scope of coupling partners is demonstrated, with multiple synthetic applications, including facile syntheses of pharmacophoric substituted N-heterocycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


