水中永久纳米气泡:液化空心碳球打破氧还原反应的极限扩散电流
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
多孔液体传统上是用空间阻碍溶剂设计的。另外,最近的研究依赖于将微孔框架分散在水等更简单的溶剂中。在这里,我们报告了一种独特的策略,通过选择性地在疏水性中空碳球(HCS)表面加入亲水性来构建大孔水。具体来说,我们表明稳定的分散表面电离HCS在水中,同时保持固有的孔隙度。水电解质中小气体分子的电催化转化受到水中气体分子浓度和扩散速率的限制。在这种情况下,大孔水的气体吸收率是无孔(纯)水的6倍。利用高容气量和增强的扩散动力学,大孔水中氧还原反应(ORR)的极限扩散电流是无孔水中的2倍,为可持续能量转换技术提供了广阔的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
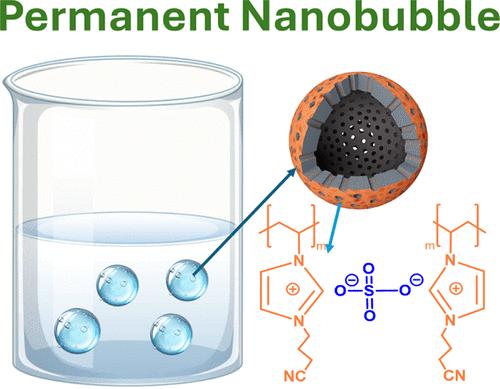
Permanent Nanobubbles in Water: Liquefied Hollow Carbon Spheres Break the Limiting Diffusion Current of Oxygen Reduction Reaction
Porous liquids have traditionally been designed with sterically hindered solvents. Alternatively, recent efforts rely on dispersing microporous frameworks in simpler solvents like water. Here we report a unique strategy to construct macroporous water by selectively incorporating hydrophilicity on the surfaces of hydrophobic hollow carbon spheres (HCS). Specifically, we show that the stable dispersion surface ionized HCS in water while retaining the inherent porosity. The electrocatalytic conversion of small gas molecules in aqueous electrolytes is limited by the concentration and diffusion rates of gas molecules in water. In this case, macroporous water exhibited 6 times gas uptake compared to nonporous (pure) water. By leveraging the high gas capacity and enhanced diffusion kinetics, the limiting diffusion current of oxygen reduction reaction (ORR) in macroporous water is 2 times that in nonporous water, offering promising prospects for sustainable energy conversion technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


