NTCDA钝化黑磷氧化过程及稳定性增强的分子动力学研究
IF 2.9
4区 工程技术
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
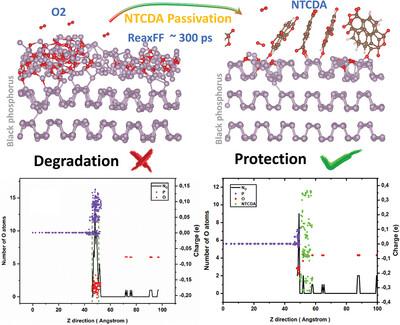
在原子尺度上了解黑磷(BP)的氧化机制对于开发有效的钝化策略以提高其在环境条件下的稳定性至关重要。为此,利用反应力场(ReaxFF)分子动力学模拟,在恒定浓度和室温条件下研究了O2和H2O分子对BP层的影响。作为一种潜在的溶液,评价了1,4,5,8-萘四羧酸二酐(NTCDA)的钝化效果。通过原子结构变化、电荷动力学和径向分布函数分析了初始氧化过程。此外,还定量确定了氧化BP层的厚度。结果表明,O2浓度的升高显著加速了氧化过程,并增加了氧化层的厚度,而H2O的影响较弱。O⁻和H⁺在水中的相互作用减少了它与BP的相互作用,但是O2分子使H2O带负电荷,使它能够与P⁺相互作用。重要的是,用NTCDA钝化BP有效地减轻氧化,形成一个保护层,排斥O2分子。最终,本研究揭示了BP层的初始氧化和钝化过程,为指导实验方法和半导体器件的实际应用提供了重要的理论见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Elucidating the Oxidation Process and Enhanced Stability of Black Phosphorus through NTCDA Passivation: A Molecular Dynamics Study
Understanding the oxidation mechanisms of black phosphorus (BP) at the atomic scale is essential for developing effective passivation strategies to enhance its stability in ambient conditions. To explore this, the effects of O2 and H2O molecules on BP layers are elucidated using reactive force field (ReaxFF) molecular dynamics simulations at constant concentrations of molecules and room temperature. As a potential solution, the passivation efficacy of 1,4,5,8-naphthalenetetracarboxylic dianhydride (NTCDA) is evaluated. The initial oxidation processes are analyzed through atomic structural changes, charge dynamics, and radial distribution functions. Moreover, the thickness of the oxidized BP layers is quantitatively determined. Results show that elevated O2 concentrations significantly accelerate oxidation and increase the thickness of the oxidized layers, while H2O has a weaker influence. The interaction between O⁻ and H⁺ ions in H2O reduces its interaction with BP, but O2 molecules cause H2O to become negatively charged, allowing it to interact with P⁺ ions. Importantly, passivating BP with NTCDA effectively mitigates oxidation, creating a protective layer that repels O2 molecules. Ultimately, this study reveals the initial oxidation and passivation processes of BP layers, offering crucial theoretical insights to guide experimental methods and practical applications in semiconductor devices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Theory and Simulations
Multidisciplinary-Multidisciplinary
CiteScore
5.50
自引率
3.00%
发文量
221
期刊介绍:
Advanced Theory and Simulations is an interdisciplinary, international, English-language journal that publishes high-quality scientific results focusing on the development and application of theoretical methods, modeling and simulation approaches in all natural science and medicine areas, including:
materials, chemistry, condensed matter physics
engineering, energy
life science, biology, medicine
atmospheric/environmental science, climate science
planetary science, astronomy, cosmology
method development, numerical methods, statistics
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


