汉氏酯催化的无金属脱氧性脱氧性吉斯加成反应
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了一种新的脱羧自由基加成反应策略,该策略在环境和露天条件下使用基态还原的烟酰胺腺嘌呤二核苷酸(NADH)类似物,促进了各种底物中Csp3-Csp3键的有效形成。该工艺具有操作简单、反应条件温和、效率高、原料成本低等特点。此外,实验研究为反应机制提供了有价值的见解,阐明了促进这些转化的不依赖光的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
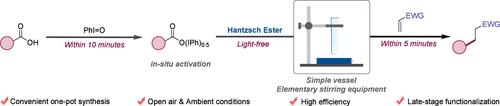
Metal- and Light-Free Decarboxylative Giese Addition Reaction Facilitated by Hantzsch Ester
We have developed a novel strategy for decarboxylative radical addition reactions that employs ground-state reduced nicotinamide adenine dinucleotide (NADH) analogues under ambient and open-air conditions, facilitating the efficient formation of Csp3–Csp3 bonds in a variety of substrates. This protocol is distinguished by its operational simplicity, mild reaction conditions, high efficiency, and the use of cost-effective starting materials. Furthermore, experimental studies have provided valuable insights into the reaction mechanism, elucidating the light-independent pathways that promote these transformations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


