通过C-C和叔C-O键形成光氧化还原催化烯烃与酰基肟酯的酰基化反应
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们描述了一种高效的烯烃酰基酯化方法,利用酰基肟酯作为双功能试剂,具有自由基酰化和拥挤的C-O键形成。该方法具有光氧化还原条件温和、台阶和原子经济性高、底物范围广、区域选择性好等特点。介绍了多种有价值的α-酰基受阻醇酯,包括通过克级合成和药物分子后期功能化获得的α-酰基受阻醇酯,展示了其合成潜力和实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
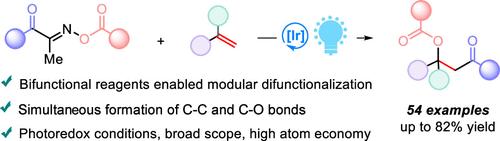
Photoredox-Catalyzed Alkene Acylesterification with Acyloxime Esters via C–C and Tertiary C–O Bond Formation
We describe an efficient acyl esterification method for alkenes utilizing acyloxime esters as bifunctional reagents featuring radical acylation and congested C–O bond formation. This approach is characterized by mild photoredox conditions, high step and atom economy, a broad substrate scope, and excellent regioselectivity. A variety of valuable α-acyl hindered alcohol esters, including those obtained via gram-scale synthesis and late-stage functionalization of pharmaceutical molecules, were presented, demonstrating its synthetic potential and practicability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


