电催化析氧中Co掺杂和Cr空位协同优化镍铁双位点原子环境
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
NiFeOOH的双位点协同催化机理表明,NiFeOOH对Ni位点的弱吸附和对Fe位点的强吸附限制了NiFeOOH对碱性析氧反应(OER)的活性。大规模密度泛函理论(DFT)计算证实,Co掺杂可以增加Ni的吸附,而金属空位可以减少Fe的吸附。这两个因素的结合可以进一步调节原子环境,优化含氧中间体的自由能,从而提高OER活性。因此,我们利用Co掺杂和Cr空位制备了VCr非晶催化剂Co- nifeooh。它在100 mA cm-2下提供239 mV的OER过电位,在500 mA cm-2下提供500小时的高稳定性,电位保留率为98%。所制备的阴离子交换膜(AEM)水电解槽在1.68 V、1 M KOH条件下具有1 a cm-2的优异性能。XPS、soft-XAS和XANES结合Bader电荷分析结果表明,局部微环境的调节可以通过Co掺杂提高Ni的价态,从而提高Ni位点上的吸附能。Cr空位可以缓解铁离子对其的强吸附。DFT计算证实,Co掺杂和Cr空位的协同作用可以使Ni/Fe位上的电荷重新分布,优化Ni和Fe的d带中心,使催化剂具有Ni - Fe双位,从而降低OER速率决定步骤的能垒。本文章由计算机程序翻译,如有差异,请以英文原文为准。
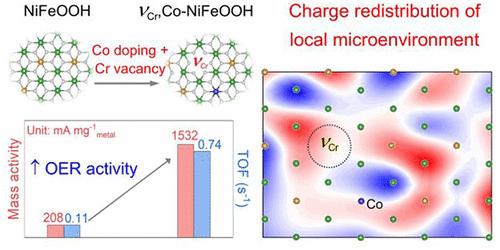
Synergistic Atomic Environment Optimization of Nickel–Iron Dual Sites by Co Doping and Cr Vacancy for Electrocatalytic Oxygen Evolution
The dual-site synergistic catalytic mechanism on NiFeOOH suggests weak adsorption of Ni sites and strong adsorption of Fe sites limited its activity toward alkaline oxygen evolution reaction (OER). Large-scale density functional theory (DFT) calculations confirm that Co doping can increase Ni adsorption, while the metal vacancy can reduce Fe adsorption. The combined two factors can further modulate the atomic environment and optimize the free energy toward oxygen-containing intermediates, thus enhancing the OER activity. Accordingly, we used Co doping and Cr vacancies to fabricate an amorphous catalyst of VCr,Co-NiFeOOH. It provides an OER overpotential of 239 mV at 100 mA cm–2 and high stability over 500 h at 500 mA cm–2 with a ∼98% potential retention. The resulting water electrolyzer based on an anion exchange membrane (AEM) exhibits a remarkable performance of 1 A cm–2 at 1.68 V in 1 M KOH. XPS, soft-XAS, and XANES combined with Bader charge analysis results reveal that the regulation of the local microenvironment can increase the valence state of Ni by Co doping, thus improving the adsorption energy on Ni sites. The Cr vacancy can alleviate the strong adsorption on Fe sites. DFT calculations confirm that the synergistic effect of Co doping and Cr vacancies can redistribute the charge on the Ni/Fe sites, optimize the d-band center of Ni and Fe, and endow the catalyst with Ni–Fe dual sites to reduce the energy barrier of the OER rate-determining step.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


