腺苷钴胺依赖酶乙醇胺解氨酶助因子钴-碳键均解机制的光谱和计算研究
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
腺苷钴胺(AdoCbl)依赖酶乙醇胺解氨酶(EAL)催化乙醇胺转化为乙醛和氨。与所有adocbl依赖的异构酶一样,EAL的催化循环是由辅因子的Co-C键的均裂裂解开始的,产生CoIIcobalamin (CoIICbl)和从底物中提取氢原子的腺苷基自由基。值得注意的是,在底物存在的情况下,酶结合AdoCbl的Co-C键均解速率提高了12个数量级。对于一类AdoCbl依赖异构体酶来说,加速速率的一个重要贡献来自于CoIICbl水解后产物的稳定,轴向协调的组氨酸残基取代了AdoCbl与这些酶结合时Co离子上的悬垂碱基。然而,EAL和其他II类异构酶以所谓的“碱基”构象结合AdoCbl,因此必须采用不同的Co-C键激活机制。在本研究中,我们使用了光谱和计算相结合的方法来探测导致EAL中Co-C键均解速率加快的构象变化和酶/辅因子/底物相互作用。AdoCbl和CoIICbl的光谱数据显示,在没有底物和存在底物的情况下,辅因子与EAL结合的扰动最小。利用分子动力学和量子力学/分子力学计算方法建立了自由AdoCbl和eal结合AdoCbl的结构模型。通过对游离和EAL结合AdoCbl的Co-C键裂解进行松弛势能扫描,我们确定了有助于EAL激活Co-C键的关键辅因子/酶相互作用,并获得了与已发表的实验数据一致的Co-C键解离能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
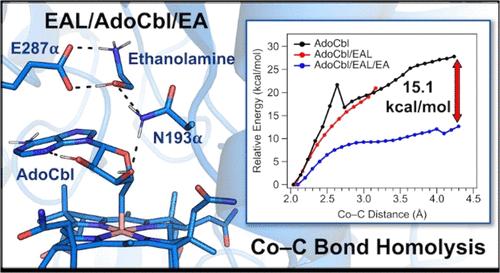
Spectroscopic and Computational Insights into the Mechanism of Cofactor Cobalt–Carbon Bond Homolysis by the Adenosylcobalamin-Dependent Enzyme Ethanolamine Ammonia-Lyase
The adenosylcobalamin (AdoCbl)-dependent enzyme ethanolamine ammonia-lyase (EAL) catalyzes the conversion of ethanolamine to acetaldehyde and ammonia. As is the case for all AdoCbl-dependent isomerases, the catalytic cycle of EAL is initiated by homolytic cleavage of the cofactor’s Co–C bond, producing CoIIcobalamin (CoIICbl) and an adenosyl radical that serves to abstract a hydrogen atom from the substrate. Remarkably, in the presence of substrate, the rate of Co–C bond homolysis of enzyme-bound AdoCbl is increased by 12 orders of magnitude. For Class I AdoCbl-dependent isomerases, an important contribution to this rate acceleration stems from a stabilization of the CoIICbl posthomolysis product by the axially coordinated histidine residue that displaces the pendant base from the Co ion upon AdoCbl binding to these enzymes. However, EAL and other Class II isomerases bind AdoCbl in the so-called “base-on” conformation and must therefore employ a different mechanism of Co–C bond activation. In the present study, we have used a combined spectroscopic and computational approach to probe the conformational changes and enzyme/cofactor/substrate interactions that contribute to the rate acceleration of Co–C bond homolysis in EAL. Spectroscopic data of AdoCbl and CoIICbl show minimal perturbations upon cofactor binding to EAL in both the absence and presence of substrate. Structural models of free and EAL-bound AdoCbl were constructed using molecular dynamics and quantum mechanics/molecular mechanics computations. By carrying out relaxed potential energy scans for Co–C bond cleavage of free and EAL-bound AdoCbl, we identified key cofactor/enzyme interactions that contribute to the Co–C bond activation by EAL and obtained Co–C bond dissociation energies that agree well with published experimental data.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


