铜/金红石- tio2(110)水解离制氢:簇大小的影响
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
cu负载型TiO2催化剂在光催化水裂解制氢和水气转换反应中表现出优异的反应活性。然而,它们的反应性的起源仍然知之甚少。在这项工作中,我们系统地研究了在Cu簇覆盖的金红石(R)-TiO2(110)表面(Cu/R-TiO2(110))上H2O解离产生H2。结果表明,Cu/R-TiO2(110)在~ 220和~ 360 K下分别发生H2O转化制氢。低温产氢可能是通过Cu团簇与界面水分子的协同催化过程进行的,高温产氢可能是通过吸附在Cu团簇上的质子结合进行的。进一步分析表明,低温产氢通道与Cu簇大小有关。本研究加深了对Cu/R-TiO2(110)上H2O转化制氢机理的认识,有助于开发新型高效制氢催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
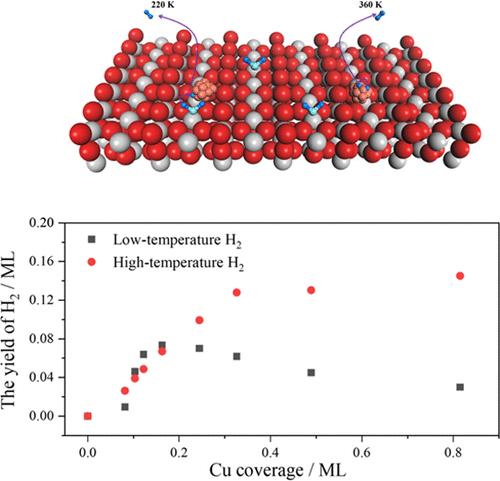
Hydrogen Production via Water Dissociation on Cu/Rutile-TiO2(110): The Effect of Cluster Size
Cu-supported TiO2 catalysts show excellent reactivity in hydrogen (H2) production from photocatalytic water (H2O) splitting and the water–gas shift reaction. However, the origin of their reactivity remains poorly understood. In this work, we have investigated H2 production from H2O dissociation on a Cu cluster-covered rutile(R)-TiO2(110) surface (Cu/R-TiO2(110)) systematically. The results show that H2 production from H2O conversion can occur on Cu/R-TiO2(110) at ∼220 and ∼360 K, respectively. The low-temperature H2 production is likely to occur via the synergistic catalysis process between Cu clusters and interfacial H2O molecules, and the high-temperature pathway occurs via the combination of protons adsorbed on the Cu clusters. Further analysis suggests that the low-temperature H2 production channel is related to Cu cluster size. This work deepens the understanding of H2 production via H2O conversion on Cu/R-TiO2(110), which will be beneficial for the development of new catalysts for efficient H2 production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


