金属氮肽:控制金属与肽主氮的螯合
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们提出了控制金属螯合肽骨架的第一种方法,其中锚定位点是偶氮氨基酸氮,螯合事件的方向性是由邻近NHs的酸度决定的。选择性主链螯合排除了对金属结合侧链和/或游离N或c端肽的需要。我们证明了氮杂氨基酸的存在和位置影响络合物的形成,并报道了氮杂肽与钯和镍结合的第一个x射线晶体结构。还提出了金属氮肽的收缩异构性的证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
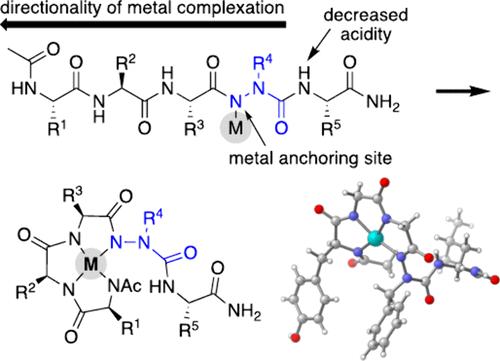
Metallo-azapeptides: Controlled Metal Chelation to Peptide Backbone Nitrogen
We present the first approach to controlled metal chelation of peptide backbones, where the anchoring site is an aza-amino acid nitrogen and the directionality of chelation events is dictated by the acidity of neighboring NHs. Selective backbone chelation precludes the need for metal-binding side chains and/or free N- or C-termini in peptides. We show that the presence and location of an aza-amino acid impact complex formation and report the first X-ray crystal structures of azapeptides bound to palladium and nickel. Evidence of atropisomerism in metallo-azapeptides is also presented.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


