手性锰配合物介导的聚烯烃高位置和对映选择性催化环氧化反应,包括角鲨烯一步转化为天然甾体和萜类前体(S)-2,3-环氧化物
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文报道了一种新的Mn(II)手性二羧基配体的合成,以及Mn配合物在温和条件下应用于C = C的高度对映和位置选择性环氧化反应,甚至使用聚烯烃底物。提出了不对称感应的立体力学基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
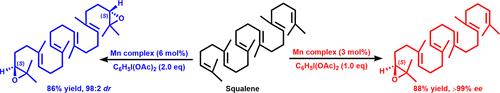
Highly Position- and Enantioselective Catalytic Epoxidation of Polyolefins Mediated by a Chiral Mn Complex, Including a One-Step Conversion of Squalene to the (S)-2,3-Epoxide, a Precursor of Natural Steroids and Terpenoids
Reported herein is the synthesis of a novel chiral dicarboxylic ligand for Mn(II) and the application of the Mn complex to the highly enantio- and position-selective epoxidation of C═C under mild conditions, even with polyolefinic substrates. A stereomechanistic basis for asymmetric induction is suggested.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


