氮腈和缺电子烯烃协同配体作用下镍催化胺中未活化烯烃的区域选择性烷基化反应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们报告了γ,δ- 和 δ,ε-烯胺与芳基卤化物和烷基锌试剂在镍催化下发生的未活化烯烃的代烷芳基化反应。该反应通过胺配位实现,可使用所有伯胺、仲胺和叔胺。该反应可构建两个新的 C(sp3)-C(sp3) 和 C(sp3)-C(sp2) 键,并生成在 γ 和 δ 位具有 C(sp3)- 支化的 δ- 和 ε- 芳胺。各种芳基和杂芳基碘化物以及伯、仲烷基锌试剂均可用作偶联碳源。机理研究表明,有机腈和作为配体的缺电子烯(EDAs)的协同作用使反应得以进行。本文章由计算机程序翻译,如有差异,请以英文原文为准。
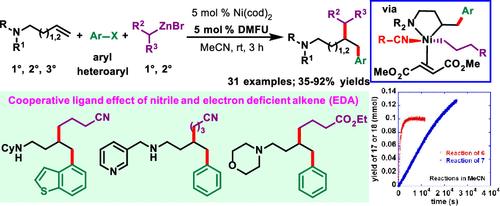
Ni-Catalyzed Regioselective Alkylarylation of Unactivated Alkenes in Amines Enabled by Cooperative Ligand Effects of Nitriles and Electron-Deficient Alkenes
We report a Ni-catalyzed vicinal alkylarylation of unactivated alkenes in γ,δ- and δ,ε-alkenylamines with aryl halides and alkylzinc reagents. The reaction is enabled by amine coordination and can use all primary, secondary, and tertiary amines. The reaction constructs two new C(sp3)–C(sp3) and C(sp3)–C(sp2) bonds and produces δ- and ε-arylamines with C(sp3)-branching at the γ- and δ-positions. A variety of aryl and heteroaryl iodides and both the primary and secondary alkylzinc reagents can be used as coupling carbon sources. Mechanistic studies suggest that the reaction is enabled by the cooperative effect of organic nitriles and electron-deficient alkenes (EDAs) as ligands.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


