应用于非水氧化还原液流电池的三苯基膦氧化物衍生阳极液
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
氧化还原液流电池的最新进展使其成为电网规模储能的可行选择,但它们的能量密度较低。提高能量密度的一种方法是使用非水系统增加电池电位。分子工程已被证明是开发极具潜力的氧化还原活性化合物的有效策略,但这通常受到新开发的氧化还原材料资源可持续性的挑战。在这里,我们研究了磷氧化物作为极负电位的阳极电解质的用途。具体来说,我们发现环三苯基氧化膦(CPO)具有很高的负电位(- 2.4 V vs Fc/Fc+)。重要的是,CPO是由氧化三苯基膦合成的,这是一种没有商业价值的常见工业化学废物。还原后的自由基阴离子的结构和电化学表征表明,稳定性的增强是由于环化或扩展的偶联。重要的是,对CPO在各种溶剂和电化学条件下分解的机理研究使我们能够利用乙腈/DMF二元溶剂体系来实现长寿命的阳极电解质,该阳极电解质在350次循环后不会褪色。总之,这项工作导致了一种废物衍生的循环氧化膦的开发,它具有优异的循环稳定性,使其成为开发能量密集rfb的理想阳极电解质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
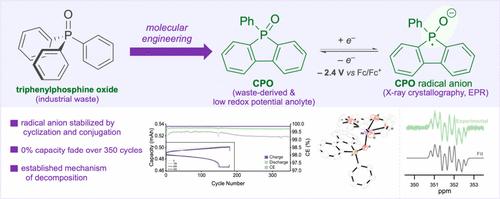
Triphenylphosphine Oxide-Derived Anolyte for Application in Nonaqueous Redox Flow Battery
Recent advances in redox flow batteries have made them a viable option for grid-scale energy storage, however they exhibit low energy density. One way to boost energy density is by increasing the cell potential using a nonaqueous system. Molecular engineering has proven to be an effective strategy to develop redox-active compounds with extreme potentials but these are usually challenged by resource sustainability of the newly developed redox materials. Here, we investigate the utility of phosphine oxides as anolytes with extremely negative potentials. Specifically, we found that cyclic triphenylphosphine oxide (CPO), has a highly negative potential (−2.4 V vs Fc/Fc+). Importantly, CPO is synthesized from triphenylphosphine oxide, a common industrial chemical waste with no commercial value. Structural and electrochemical characterization of the reduced radical anion showed that enhanced stability is due to cyclization or extended pi-conjugation. Importantly, mechanistic investigation into the decomposition of CPO under various solvents and electrochemical conditions allowed us to utilize an acetonitrile/DMF binary solvent system to enable a long-lived anolyte which exhibited no fade over 350 cycles. In summary, this work led to the development of a waste-derived cyclic phosphine oxide that exhibits excellent cycling stability making it an ideal anolyte toward the development of energy-dense RFBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


