多重细胞表面蛋白标记的单生物正交反应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
小分子荧光团是荧光成像的宝贵工具。然而,它们与靶蛋白共价结合的手段限制了它们在多色成像中的应用。在这里,我们通过详细的机理研究确定了2-[(烷基硫基)(芳基)亚甲基]丙二腈(TAMM)分子与1,2-氨基硫醇以超过104 M-1 s-1的标记速率反应。快速的TAMM分子和温和的反应条件使其不仅可以在细胞系中,而且可以在原代神经元和活体小鼠中进行表面蛋白的位点特异性标记。遗传密码扩展和序列特异性蛋白水解裂解相结合,可以通过迭代TAMM冷凝对三种不同的细胞表面蛋白进行选择性修饰。TAMM缩合也与cu催化叠氮-炔环加成和四嗪连接相兼容,用于四色荧光标记,达到常规共聚焦显微镜的最大可用颜色。因此,生物偶联化学不再是多重细胞表面蛋白成像的限制因素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
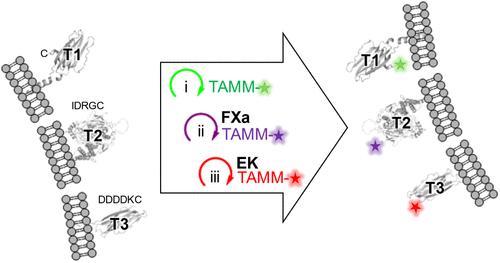
A Single Bioorthogonal Reaction for Multiplex Cell Surface Protein Labeling
Small-molecule fluorophores are invaluable tools for fluorescence imaging. However, means for their covalent conjugation to the target proteins limit applications in multicolor imaging. Here, we identify 2-[(alkylthio)(aryl)methylene]malononitrile (TAMM) molecules reacting with 1,2-aminothiol at a labeling rate over 104 M–1 s–1 through detailed mechanistic investigation. The fast TAMM molecules and mild reaction conditions enable site-specific labeling of surface proteins in not only cell lines but also primary neurons and living mice. The combination of genetic code expansion and sequence-specific proteolytic cleavage enables selective modification of three different cell surface proteins through iterative TAMM condensation. TAMM condensation is also compatible with Cu-catalyzed azide–alkyne cycloaddition and tetrazine ligation for four-color fluorescent labeling, reaching the maximum available colors of conventional confocal microscopes. Thus, bioconjugation chemistry is no longer the limiting factor for multiplex cell surface protein imaging.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


