通过动态共价化学的选择性蛋白修饰:自激活SNAr反应
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
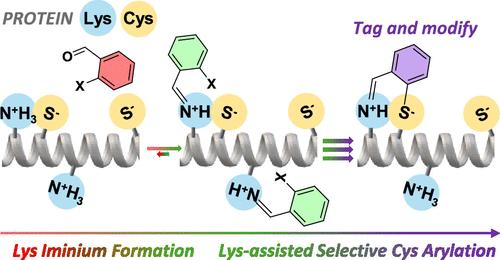
使用基于动态共价化学(DCvC)的预靶向和激活单元可显著加速SNAr反应。水杨醛衍生物芳香环上的C-F键上的Cys攻击仅在模型小肽与相邻的Lys残基形成时观察到。这种自激活归因于相对于母体醛的更强的吸电子能力,稳定了反应的过渡状态,以及阳离子铝中活性基团的预组织率更高。该方法进一步应用于两种抗体的功能化。在这两种情况下,醛基在活性C-F键附近的存在导致生物偶联率显著增加,具有优异的化学选择性。而IgG1抗体的修饰导致了随机产物分布,当使用IgG4抗体时,微环境选择性被注意到,这与后者的铰链区域的赖氨酸残基数量较少一致。此外,修饰抗体的后功能化是通过栓系的亚胺衍生物与酰肼的动态共价交换实现的,代表了一种前所未有的基于DCvC的“标记和修饰”选择性生物偶联策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Selective Protein (Post-)modifications through Dynamic Covalent Chemistry: Self-activated SNAr Reactions
SNAr reactions were remarkably accelerated using a pretargeting and activating unit based on dynamic covalent chemistry (DCvC). A Cys attack at the C–F bond on the aromatic ring of salicylaldehyde derivatives was only observed upon iminium formation with a neighboring Lys residue of model small peptides. Such self-activation was ascribed to the stronger electron-withdrawing capability of the iminium bond with respect to that of the parent aldehyde that stabilized the transition state of the reaction, together with the higher preorganization of the reactive groups in the cationic aldiminium species. This approach was further applied for the functionalization of two antibodies. In both cases, the presence of the aldehyde group in close proximity to the reactive C–F bond resulted in a noteworthy increase in bioconjugation yields, with excellent chemo-selectivity. Whereas the modification of an IgG1 antibody led to stochastic product distributions, microenvironment selectivity was noted when employing IgG4, in line with the lower number of Lys residues in the hinge region of the latter. Additionally, the postfunctionalization of the modified antibodies was attained through the dynamic covalent exchange of the tethered iminium derivative with hydrazides, representing an unprecedented “tag and modify” selective bioconjugation strategy based on DCvC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


