不同金属酞菁热解石墨壳的原子尺度原位自催化生长
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
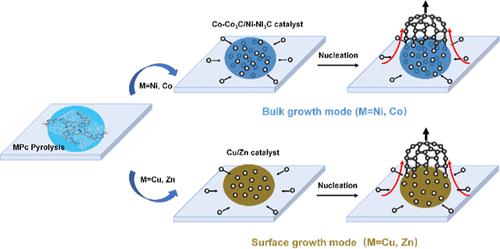
碳纳米材料作为一种具有独特性能的新型功能和结构材料,在信息技术和纳米器件领域具有重要的应用潜力。然而,碳源石墨壳在不同催化剂作用下的自催化生长机理还有待进一步深入研究。为了解决这一挑战,我们进行了一项研究,利用环境透射电子显微镜(ETEM)在原子分辨率下研究含有各种中心金属(包括Ni, Co, Cu和Zn)的金属酞菁(MPc)化合物的热解。结果表明,碳原子最初溶解到Ni和Co中,导致金属碳化物的形成,随后起到催化剂的作用。在达到碳饱和后,这些原子从内部向外沉淀,通过块状生长模式促进石墨壳的生长。相反,对于Cu和Zn,碳原子在金属表面迁移积累,导致石墨壳通过表面生长方式生长。不同的生长机制,即体生长和表面生长,是由碳原子和催化剂之间的结合能以及碳在金属中的溶解度复杂地决定的。这些因素与金属d电子的数量和能量密切相关,因为更多的空d轨道容易形成更多的配位数。这些见解为金属催化石墨壳生长的基本原理提供了深刻的理解,并为选择和设计催化剂提供了战略路线图,这些催化剂可以优化所需碳纳米材料的合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Atomic-Scale In Situ Self-Catalysis Growth of Graphite Shells via Pyrolysis of Various Metal Phthalocyanines
Carbon nanomaterials, as novel functional and structural materials with unique properties, hold significant potential in information technology and nanodevices. However, the self-catalytic growth mechanism of graphite shells derived from carbon sources, facilitated by diverse catalysts, necessitates further exhaustive investigation. To tackle this challenge, we conducted a study utilizing environmental transmission electron microscopy (ETEM) at atomic resolution to investigate the pyrolysis of metal phthalocyanine (MPc) compounds containing various central metals, including Ni, Co, Cu, and Zn. The results reveal that carbon atoms initially dissolve into Ni and Co, leading to the formation of metal carbides that subsequently act as catalysts. Upon achieving carbon saturation, these atoms precipitate outward from the interior, fostering the growth of graphite shells through a bulk growth mode. In contrast, for Cu and Zn, carbon atoms migrate and accumulate on the metal surface, resulting in the growth of graphite shells via a surface growth mode. The distinct growth mechanisms, i.e., bulk growth and surface growth, are intricately governed by the binding energy between carbon atoms and the catalysts, as well as the carbon solubility in the metal. These factors are closely tied to the number and energy of the metals’ d-electrons because more vacant d-orbitals easily forming more coordination numbers. These insights offer profound understanding into the fundamental principles of metal-catalyzed graphite shell growth and provide a strategic roadmap for selecting and designing catalysts that can can optimize the synthesis of desirable carbon nanomaterials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


