外源性丙酮酸在人体自然杀伤细胞代谢中的关键作用
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
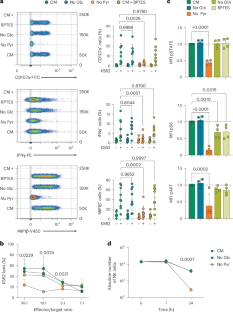
静止的自然杀伤(NK)细胞在识别转化或感染的细胞后立即表现出效应功能。维持这些功能的环境营养素和代谢需求尚未完全了解。在这里,我们表明NK细胞依赖于使用细胞外丙酮酸来支持效应功能、信号转导和细胞活力。葡萄糖衍生的碳不产生内源性丙酮酸。因此,NK细胞输入胞外丙酮酸,丙酮酸被还原成乳酸再生糖酵解NAD+,并在三羧酸(TCA)循环中氧化产生ATP。这支持通过磷酸甘油脱氢酶产生丝氨酸,这是细胞因子刺激后最佳增殖所必需的途径,但对于效应功能是必不可少的。此外,像小鼠NK细胞一样,人类NK细胞依赖于TCA循环的柠檬酸盐-苹果酸盐结构,而不是由谷氨酰胺提供。此外,生理上的丙酮酸浓度剂量依赖性地增加NK细胞的效应功能。总的来说,这项研究强调了外源性丙酮酸在NK细胞生物学中的作用,为在治疗环境中提高NK细胞的潜力提供了知识。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Pivotal role of exogenous pyruvate in human natural killer cell metabolism
Resting natural killer (NK) cells display immediate effector functions after recognizing transformed or infected cells. The environmental nutrients and metabolic requirements to sustain these functions are not fully understood. Here, we show that NK cells rely on the use of extracellular pyruvate to support effector functions, signal transduction and cell viability. Glucose-derived carbons do not generate endogenous pyruvate. Consequently, NK cells import extracellular pyruvate that is reduced to lactate to regenerate glycolytic NAD+ and is oxidized in the tricarboxylic acid (TCA) cycle to produce ATP. This supports serine production through phosphoglycerate dehydrogenase, a pathway required for optimal proliferation following cytokine stimulation but dispensable for effector functions. In addition, like mouse NK cells, human NK cells rely on a citrate–malate configuration of the TCA cycle that is not fed by glutamine. Moreover, supraphysiologic pyruvate concentrations dose-dependently increase the effector functions of NK cells. Overall, this study highlights the role of exogenous pyruvate in NK cell biology, providing knowledge that could be exploited to boost NK cell potential in therapeutic settings. Kern Coquillat et al. show that NK cells rely on exogenous pyruvate import to support effector functions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


