中性粒细胞胞外陷阱通过扩大表达IL-10的先天样B细胞,促进网膜转移前生态位的形成
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
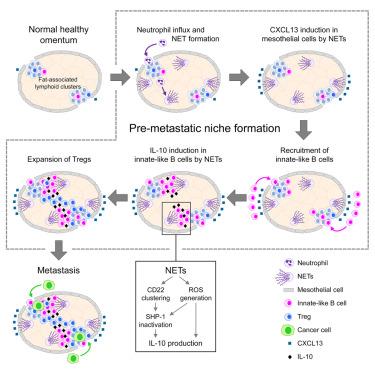
腹膜液中弥散性癌细胞常定植于大网膜脂肪相关淋巴细胞簇,但其机制尚不清楚。在这里,我们发现先天性样B细胞在患有早期卵巢癌的小鼠和女性的网膜中积累,同时被称为中性粒细胞胞外陷阱(NETs)的中性粒细胞挤压染色质纤维。利用基因修饰的net缺陷小鼠、NETs的药理抑制和过继性B细胞转移进行的研究表明,NETs诱导转移前大网膜中化学引诱物CXCL13的表达,刺激腹膜先天样B细胞的募集,进而通过产生白细胞介素(IL)-10促进调节性T细胞的扩增和大网膜转移。体外研究表明,NETs通过灭活SHP-1(一种抑制B细胞激活途径的磷酸酶)和产生活性氧,在先天样B细胞中诱导IL-10的产生。这些发现表明,NETs改变了转移前网膜中的免疫细胞动力学,使该生态位有利于定植。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Neutrophil extracellular traps promote pre-metastatic niche formation in the omentum by expanding innate-like B cells that express IL-10
Disseminated cancer cells in the peritoneal fluid often colonize omental fat-associated lymphoid clusters but the mechanisms are unclear. Here, we identify that innate-like B cells accumulate in the omentum of mice and women with early-stage ovarian cancer concomitantly with the extrusion of chromatin fibers by neutrophils called neutrophil extracellular traps (NETs). Studies using genetically modified NET-deficient mice, pharmacologic inhibition of NETs, and adoptive B cell transfer show that NETs induce expression of the chemoattractant CXCL13 in the pre-metastatic omentum, stimulating recruitment of peritoneal innate-like B cells that in turn promote expansion of regulatory T cells and omental metastasis through producing interleukin (IL)-10. Ex vivo studies show that NETs elicit IL-10 production in innate-like B cells by inactivating SHP-1, a phosphatase that inhibits B cell activation pathways, and by generating reactive oxygen species. These findings reveal that NETs alter immune cell dynamics in the pre-metastatic omentum, rendering this niche conducive for colonization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


