富锂阴极材料中抑制阴离子二聚化的金属配体自旋锁策略
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
阴离子二聚化对富锂阴极材料(lcm)在高能量密度锂离子电池中的应用提出了重大挑战,因为阴离子二聚化具有容量和电压衰减快、反应动力学缓慢和电压滞后大等不利影响。在此,我们提出了一种金属配体自旋锁策略来抑制阴离子二聚化,该策略涉及在LCM中引入具有反铁磁超交换相互作用的Fe-Ni对,以锁定阴离子中未配对电子的自旋方向相同。作为概念验证,我们将该策略应用于层内无序Li2TiS3 (ID-LTS)以抑制S-S二聚化。利用恒流充放电和间歇滴定技术进行的电化学表征表明,fe - ni偶联掺杂的ID-LTS的阴离子氧化还原活性显著增强,电压滞后降低,动力学改善。Fe L2、3边x射线吸收光谱和磁化率测量表明,金属-配体自旋锁效应和随之而来的阴离子二聚化抑制涉及到S和Fe之间的配体-金属电荷转移。对Fe - ni偶联富锂层状氧化物(Li0.7Li0.1Fe0.2Ni0.1Mn0.6O2)的进一步电化学测试表明,π背键在促进S到Fe的配体到金属电荷转移方面具有重要作用。这些发现证明了我们的金属配体自旋锁策略在高性能lcm开发中的潜在应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
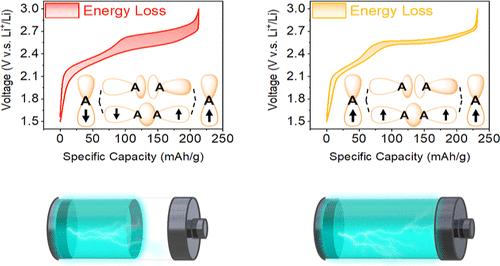
Metal–Ligand Spin-Lock Strategy for Inhibiting Anion Dimerization in Li-Rich Cathode Materials
Anion dimerization poses a significant challenge for the application of Li-rich cathode materials (LCMs) in high-energy-density Li-ion batteries because of its deleterious effects, including rapid capacity and voltage decay, sluggish reaction kinetics, and large voltage hysteresis. Herein, we propose a metal–ligand spin-lock strategy to inhibit anion dimerization, which involves introducing an Fe–Ni couple having antiferromagnetic superexchange interaction into the LCM to lock the spin orientations of the unpaired electrons in the anions in the same direction. As proof of concept, we applied this strategy to intralayer disordered Li2TiS3 (ID-LTS) to inhibit S–S dimerization. Electrochemical characterization using the galvanostatic charge/discharge and intermittent titration technique demonstrated the considerably enhanced anionic redox activity, reduced voltage hysteresis, and improved kinetics of the Fe–Ni-couple-incorporated ID-LTS. Fe L2,3-edge X-ray absorption spectroscopy and magnetic susceptibility measurements revealed that the metal–ligand spin-lock effect and consequent suppression of anion dimerization involve ligand-to-metal charge transfer between S and Fe. Further electrochemical tests on a Fe–Ni-couple-incorporated Li-rich layered oxide (Li0.7Li0.1Fe0.2Ni0.1Mn0.6O2) indicated the importance of the π backbond in enhancing ligand-to-metal charge transfer from S to Fe. These findings demonstrate the potential application of our metal–ligand spin-lock strategy in the development of high-performance LCMs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


