助溶剂电解质促进烷基醇电化学半加氢反应
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
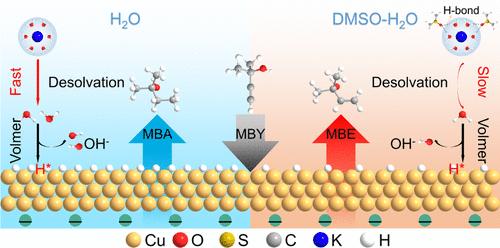
通过电催化炔醇半加氢技术实现绿色电力驱动的烯醇电合成是一种替代传统热催化的可持续途径。电催化剂和电解质对半加氢性能都有很大影响。尽管在开发复杂的电催化剂方面取得了重大进展,但设计良好的电解质与工业催化剂相结合是推进电催化炔醇半加氢工业化进程的一个有吸引力的策略,但仍未得到探索。在此,我们开发了一种用于电催化烷基醇半加氢的二甲基亚砜(DMSO)-H2O共溶剂电解质。在烷基醇转化率约为100%时,与不含dmso的电解质相比,DMSO-H2O电解质使Cu催化剂上的烷基醇选择性从60-70%提高到90%以上。同时,由于水解离受到抑制,反应速率略有降低。机理研究表明,DMSO与H2O之间的强氢键相互作用抑制了界面H2O的解离,导致电极表面H*覆盖率下降。H*覆盖率的降低阻碍了烷基醇的过氢化反应,有利于烷基醇的生成。值得注意的是,dmso诱导的烷基醇选择性增强适用于一组商业催化剂和各种烷基醇的半加氢反应。最后,建立了一个放大的3 × 100 cm2的电解槽堆,以实现在共溶剂电解质中烷基醇转化率为~ 96%,烷基醇选择性为~ 95%。这项工作不仅提出了一种促进烯醇电合成的电解质策略,而且强调了可持续烯醇电生产的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A Cosolvent Electrolyte Boosting Electrochemical Alkynol Semihydrogenation
Green electricity-driven alkenol electrosynthesis via electrocatalytic alkynol semihydrogenation represents a sustainable route to conventional thermocatalysis. Both the electrocatalyst and electrolyte strongly impact the semihydrogenation performance. Despite significant progress in developing sophisticated electrocatalysts, a well-designed electrolyte in conjunction with industrial catalysts is an attractive strategy to advance the industrialization process of electrocatalytic alkynol semihydrogenation, but remains unexplored. Here, we develop a dimethyl sulfoxide (DMSO)-H2O cosolvent electrolyte for electrocatalytic alkynol semihydrogenation. At an alkynol conversion of about 100%, the DMSO-H2O electrolyte compared to the DMSO-free counterpart enables the alkenol selectivity on Cu catalysts to be promoted from 60–70% to over 90% at all measured current densities; meanwhile, the reaction rate is slightly decreased due to the inhibited water dissociation. Mechanistic studies reveal that the strong hydrogen-bond interactions between DMSO and H2O suppress the dissociation of interfacial H2O, leading to a decreased H* coverage at the electrode surface. The decreased H* coverage hinders the overhydrogenation of alkynols and favors the production of alkenols. Remarkably, the DMSO-induced enhancement of alkenol selectivity is applicable to a set of commercial catalysts and to the semihydrogenation of various alkynols. Eventually, a scaled-up 3 × 100 cm2 electrolyzer stack is established to achieve an alkynol conversion of ∼96% and an alkenol selectivity of ∼95% in the cosolvent electrolyte. This work not only presents an electrolyte strategy for boosting alkenol electrosynthesis, but also highlights the possibility of sustainable alkenol electro-production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


