琥珀酸盐纳米材料通过激活细胞焦亡和增强MHC-I表达来促进肿瘤免疫治疗
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
尽管免疫疗法的临床应用前景广阔,但其有效性往往受到低免疫反应和肿瘤免疫逃逸的限制。在这项研究中,我们介绍了一种简单且无药物的无机纳米材料琥珀酸钠(C4H4Na2O4 NPs),采用快速微乳法制备,以增强癌症免疫治疗。合成的C4H4Na2O4 NPs可以释放高浓度的Na+和琥珀酸离子进入肿瘤细胞,导致细胞内渗透压升高。这触发了焦亡途径,导致细胞内容物、炎症因子和损伤相关分子模式的释放,最终增强免疫反应。此外,C4H4Na2O4 NPs通过上调主要组织相容性复合体i (MHC-I)的表达来抑制肿瘤免疫逃逸。综上所述,C4H4Na2O4 NPs通过焦热诱导的免疫激活和MHC-I表达上调介导肿瘤免疫逃逸,显著抑制肿瘤生长和转移。本研究为肿瘤治疗提供了一种利用MHC-I表达和焦亡的新方法,展示了在癌症免疫治疗中的临床应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
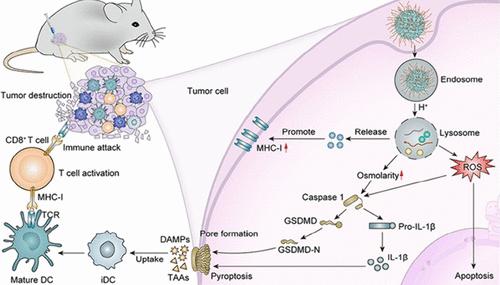
Succinate Nanomaterials Boost Tumor Immunotherapy via Activating Cell Pyroptosis and Enhancing MHC-I Expression
Despite the promising clinical applications of immunotherapy, its effectiveness is often limited by low immune responses and tumor immune escape. In this study, we introduce a simple and drug-free inorganic nanomaterial, sodium succinate (C4H4Na2O4 NPs), prepared using a rapid microemulsion method to enhance cancer immunotherapy. The synthesized C4H4Na2O4 NPs can release high concentrations of Na+ and succinate ions into tumor cells, leading to an increase in intracellular osmolarity. This triggers the pyroptosis pathway, resulting in the release of cellular contents, inflammatory factors, and damage-associated molecular patterns, which ultimately boost immune responses. Furthermore, C4H4Na2O4 NPs inhibit tumor immune escape through upregulating major histocompatibility complex-I (MHC-I) expression. Collectively, C4H4Na2O4 NPs significantly inhibit tumor growth and metastasis by pyroptosis-induced immune activation and MHC-I expression upregulation-remitted tumor immune escape. This research offers a novel approach to tumor treatment that leverages MHC-I expression and pyroptosis, demonstrating the potential for clinical application in cancer immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


