浸润浆细胞通过IgG-Tumor结合维持胶质母细胞瘤干细胞
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
胶质母细胞瘤是一种高度侵袭性的原发性脑肿瘤,胶质母细胞瘤干细胞(GSCs)加强了肿瘤内的等级。浆细胞(PCs)是b系免疫系统的关键效应器,但它们在胶质母细胞瘤中的作用仍未得到充分研究。在这里,我们利用肿瘤浸润B系细胞的单细胞RNA和B细胞受体测序,揭示了pc在胶质母细胞瘤浸润B系人群中异常富集,经历低水平的体细胞超突变,并与不良预后相关。PCs分泌免疫球蛋白G (IgG),通过IgG- fc - γ riia - akt - mtor轴刺激GSC增殖。破坏igg - fc - γ - riia旁分泌通讯抑制GSC增殖和自我更新。胶质母细胞瘤浸润性pc通过CCL2-CCR2趋化因子程序被招募到GSC壁龛。GSCs进一步通过FcγRIIA信号从广泛使用的基于单克隆抗体的免疫检查点抑制剂中获得促增殖信号。我们的数据生成了胶质母细胞瘤中b系细胞的图谱,该图谱具有肿瘤细胞内在和微环境依赖性的组合靶向框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
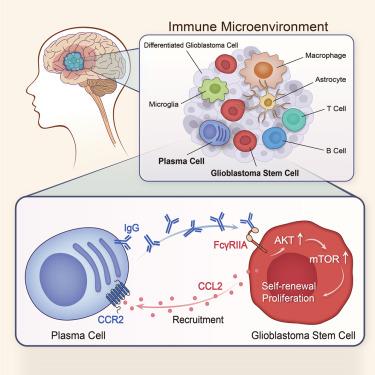
Infiltrating plasma cells maintain glioblastoma stem cells through IgG-Tumor binding
Glioblastoma is a highly aggressive primary brain tumor with glioblastoma stem cells (GSCs) enforcing the intra-tumoral hierarchy. Plasma cells (PCs) are critical effectors of the B-lineage immune system, but their roles in glioblastoma remain largely unexplored. Here, we leverage single-cell RNA and B cell receptor sequencing of tumor-infiltrating B-lineage cells and reveal that PCs are aberrantly enriched in the glioblastoma-infiltrating B-lineage population, experience low level of somatic hypermutation, and are associated with poor prognosis. PCs secrete immunoglobulin G (IgG), which stimulates GSC proliferation via the IgG-FcγRIIA-AKT-mTOR axis. Disruption of IgG-FcγRIIA paracrine communication inhibits GSC proliferation and self-renewal. Glioblastoma-infiltrating PCs are recruited to GSC niches via CCL2-CCR2 chemokine program. GSCs further derive pro-proliferative signals from broadly utilized monoclonal antibody-based immune checkpoint inhibitors via FcγRIIA signaling. Our data generate an atlas of B-lineage cells in glioblastoma with a framework for combinatorial targeting of both tumor cell-intrinsic and microenvironmental dependencies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


