多氟烷酸作为氟烷基化试剂:直接获取rf包埋酰胺的策略
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了在无金属条件下,将稳定的全氟烷酸作为氟烷基化试剂,与DIB和伯胺结合,连续一锅转化为rf嵌入的功能化酰胺。该方案可耐受一系列敏感官能团(33例,产率高达90%)和全氟酸。通过初步的机理研究、对照实验、原位19F-NMR分析和中间物质的合成来了解反应途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
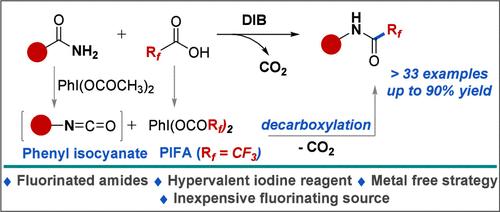
Polyfluoroalkanoic Acids as Fluoroalkylating Reagents: Strategy for Direct Access to Rf-Embedded Amides
Herein, we have reported the application of bench stable perfluoroalkanoic acids as fluoro-alkylating reagents in combination with DIB and primary amides for sequential one-pot transformation to Rf-embedded functionalized amides under metal-free conditions. The protocol is tolerant to a range of sensitive functional groups (>33 examples and up to 90% yield), and perfluoro acids. Preliminary mechanistic studies, control experiments, in situ 19F-NMR analyses, and the synthesis of intermediate species were performed to understand the reaction pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


