c1功能化低聚糖的自动合成
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
自动聚糖组装(AGA)简化了复杂低聚糖的合成。低聚糖的还原端作为聚合物载体的附着位点,以释放游离的还原端或氨基戊醇,以便与载体蛋白或表面偶联。到目前为止,还不可能通过AGA在低聚糖上方便地安装不同的苷元。在这里,我们描述了一种由无迹可光性连接器实现的潜在主动方法,该连接器允许双向AGA和随时引入各种苷元。低聚糖、肽聚糖、原型皂苷和基于点击化学的偶联物被合成,以说明该方法的多功能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
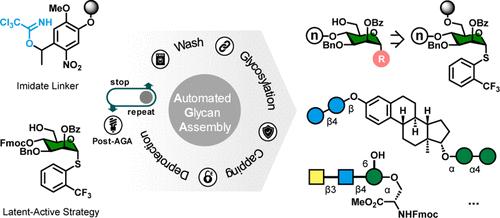
Automated Synthesis of C1-Functionalized Oligosaccharides
Automated glycan assembly (AGA) streamlines the synthesis of complex oligosaccharides. The reducing end of the oligosaccharide serves as an attachment site to the polymer support to liberate a free reducing end or an aminopentanol for ready conjugation to carrier proteins or surfaces. The facile installation of different aglycons on oligosaccharides has not been possible via AGA until now. Here, we describe a latent-active approach enabled by a traceless photolabile linker that allows for bidirectional AGA and ready introduction of various aglycons. Oligosaccharide thioglycosides, peptidoglycans, prototypical saponins, and click-chemistry-based conjugates are synthesized to illustrate the versatility of the method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


