内酰胺对映选择性β-C(sp3) -H活化合成含四元中心的手性饱和杂环
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
对映纯饱和杂环的催化合成方法的发展一直是不对称催化领域的一个长期挑战。我们描述了第一个高度对映选择性钯催化内酰胺的β-C(sp3) -H芳基化和烯烃化,用于制备各种手性n -杂环,含季碳中心。手性双功能MPAThio配体上强吸电子基团的存在对弱配位内酰胺的反应性至关重要。由此产生的对映体富集内酰胺很容易转化为手性哌啶和亚胺家族,这在药物发现中是非常理想的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
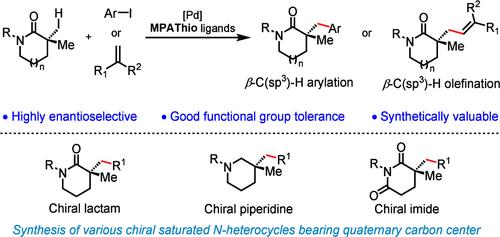
Synthesis of Chiral Saturated Heterocycles Bearing Quaternary Centers via Enantioselective β-C(sp3)–H Activation of Lactams
The development of catalytic methods for the synthesis of enantiopure saturated heterocycles has been a long-standing challenge in asymmetric catalysis. We describe the first highly enantioselective palladium-catalyzed β-C(sp3)–H arylation and olefination of lactams for the preparation of various chiral N-heterocycles bearing quaternary carbon centers. The presence of strongly electron-withdrawing groups on the chiral bifunctional MPAThio ligand is crucial to the reactivity of weakly coordinating lactams. The resulting enantioenriched lactams are readily converted to a family of chiral piperidines and imides that are highly desirable in drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


