软海绵素B的大环酮类类似物依瑞布林的普林斯大环合成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过反复的砜片段偶联,以及涉及C.26全烯醇和C.27醛缩醛的分子内Prins反应,实现了完全无铬合成eribulin,这是一种海洋天然产物软海绵素B的完全合成的大环酮类似物。这种新的大环化的一个显著特征是在C.15/14处使用β-酮砜作为众所周知的酸敏感多环酮部分的酸稳定前体,这是软海绵素的特征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
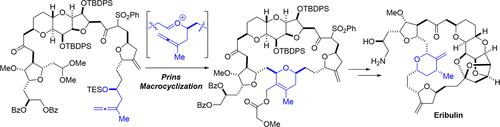
Total Synthesis of Eribulin, a Macrocyclic Ketone Analogue of Halichondrin B, via Prins Macrocyclization
An entirely chromium-free synthesis of eribulin, a fully synthetic macrocyclic ketone analogue of the marine natural product halichondrin B, was achieved through iterative sulfone fragment couplings followed by an intramolecular Prins reaction involving a C.26 homoallenyl alcohol and a C.27 aldehyde acetal. A notable feature of this new macrocyclization is the employment of a β-ketosulfone at C.15/14 as an acid-stable progenitor of the notoriously acid-sensitive polycyclic ketal moiety, characteristic of the halichondrins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


