Ca2+/钙调素依赖性蛋白激酶II β解码ER Ca2+瞬态触发自噬体形成
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在多细胞生物中,对于内质网外表面由自噬刺激引起的Ca2+瞬态如何持续和解码以触发自噬体形成知之甚少。在这里,我们发现Ca2+/钙调素依赖性蛋白激酶IIβ (CaMKIIβ)整合ER Ca2+瞬态来触发自噬体启动的FIP200复合物的液-液相分离(LLPS)。在响应ER Ca2+瞬态时,CaMKIIβ从肌动蛋白丝中招募并形成凝聚体,这是FIP200点出现或与FIP200点相互作用的位点。CaMKIIβ磷酸化FIP200的Thr269、Thr1127和Ser1484位点,从而调节FIP200复合物的LLPS和特性,从而控制其在自噬体形成中的功能。CaMKIIβ还控制自噬诱导过程中ER Ca2+瞬态的振幅、持续时间和传播。在神经发育障碍MRD54中发现的CaMKIIβ突变影响CaMKIIβ在自噬中的功能。我们的研究表明,CaMKIIβ对于维持和解码哺乳动物细胞中ER Ca2+瞬态以指定自噬体形成至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
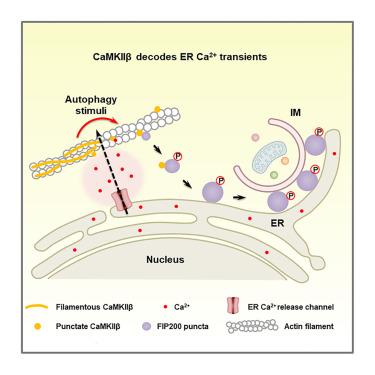
Ca2+/calmodulin-dependent protein kinase II β decodes ER Ca2+ transients to trigger autophagosome formation
In multicellular organisms, very little is known about how Ca2+ transients on the ER outer surface elicited by autophagy stimuli are sustained and decoded to trigger autophagosome formation. Here, we show that Ca2+/calmodulin-dependent protein kinase II β (CaMKIIβ) integrates ER Ca2+ transients to trigger liquid-liquid phase separation (LLPS) of the autophagosome-initiating FIP200 complex. In response to ER Ca2+ transients, CaMKIIβ is recruited from actin filaments and forms condensates, which serve as sites for the emergence of or interaction with FIP200 puncta. CaMKIIβ phosphorylates FIP200 at Thr269, Thr1127, and Ser1484 to modulate LLPS and properties of the FIP200 complex, thereby controlling its function in autophagosome formation. CaMKIIβ also controls the amplitude, duration, and propagation of ER Ca2+ transients during autophagy induction. CaMKIIβ mutations identified in the neurodevelopmental disorder MRD54 affect the function of CaMKIIβ in autophagy. Our study reveals that CaMKIIβ is essential for sustaining and decoding ER Ca2+ transients to specify autophagosome formation in mammalian cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


