α-羰基混合催化对映选择性合成1,4-(杂)二羰基化合物
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
1,4-二羰基化合物的对映选择性合成仍然是有机合成中的一个重大挑战,迄今为止还缺乏一种能够生成两个相邻立体中心并具有完全立体化学控制的催化过程。官能团之间的1,4关系需要Umpolung策略,因为α-羰基的一个位置必须反转成亲电中心才能与正常的烯醇酯反应。我们在此报道了高对映性和非对映选择性的硅基烯酮缩醛向亲电的1-氮杂烯基阳离子加成,得到手性4-腙酯,这是一种被掩盖的1,4-二羰基化合物。产物携带多达2个新的立体中心,在广泛的底物范围内获得了优异的产量。α-乙酰氧基腙作为1-氮杂烯基阳离子的前体,用强刘易斯酸手性酰亚胺二磷酰咪酯(IDPi)电离。得到的离子对通过核磁共振和质谱进行了表征,而DFT计算提供了对反应机理的进一步了解。此外,通过合成抗惊厥剂普瑞巴林和布瓦西坦前体,这些产物成功地转化为对映体上高度富集的b-氰基和b-甲酰基酯以及γ-内酰胺和γ-氨基酸。本文章由计算机程序翻译,如有差异,请以英文原文为准。
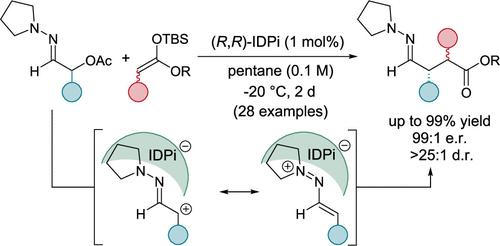
Catalytic Enantioselective Synthesis of 1,4-(Hetero) Dicarbonyl Compounds through α-Carbonyl Umpolung
The enantioselective synthesis of 1,4-dicarbonyl compounds continues to pose a significant challenge in organic synthesis, and a catalytic process which generates two adjacent stereogenic centers with full stereochemical control is lacking until now. The 1,4-relationship of the functional groups requires an Umpolung strategy as one of the α-carbonyl positions has to be inverted into an electrophilic center to react with a normal enolate. We report herein the highly enantio- and diastereoselective addition of silyl ketene acetals toward electrophilic 1-azaallyl cations to furnish chiral 4-hydrazonoesters, which are masked 1,4-dicarbonyl compounds. The products carrying up to 2 new stereogenic centers were obtained in excellent yields across a broad substrate scope. As precursors to the 1-azaallyl cations, α-acetoxy hydrazones were employed and ionized with a strongly Lewis acidic, chiral silylium imidodiphosphorimidate (IDPi). The resulting ion pair was characterized with NMR and mass spectroscopy, while DFT calculations provided further insights into the reaction mechanism. In addition, the products were successfully converted into enantiomerically highly enriched b-cyano and b-formyl esters as well as γ-lactams and γ-amino acids, as demonstrated by syntheses of the anticonvulsant agent pregabalin and a brivaracetam precursor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


