羧酸光催化活化中的自由基中间体:自旋俘获研究
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
采用DBNBS(3,5-二溴-4-亚硝基苯磺酸钠盐)自旋捕获剂捕获羧酸与负载铂的TiO2光催化剂光催化反应产生的自由基,并用电子自旋共振光谱对自由基加合物进行了观察。在乙酸和丙酸在水和乙腈的混合溶剂中,由于脱羧反应,只观察到甲基和乙基自由基的加合物。线性烷基羧酸的烷基链越长,自由基加合物的比例越大,总加合物的浓度越高。结果表明,随着烷基链长度的增加,自由基的扩散速度减慢。此外,在扩散过程中,分子内的氢转移反应发生。这些反应的发生速率比用自旋捕获试剂的反应速率要高。电喷雾电离质谱(ESI-MS)不仅可以检测十一烷基自由基的加合物,还可以检测十二烷酸自由基的加合物。表明长烷基羧酸的自由基阳离子([HRCOOH]•+)脱羧生成烷基自由基(HR•)或质子消除生成烷基羧酸自由基(•RCOOH)的竞争性反应是由于自由基阳离子通过与较长烷基链的超共轭而稳定以及溶剂中存在水作为质子受体而发生的。具有叔碳的支链烷基羧酸也是如此,它稳定了自由基阳离子。虽然这一结果也暗示了由于产物自由基之间的耦合和缺乏产物选择性而增加了产物多样性,但我们发现只有当溶剂中水的百分比降低到5%以下时,脱羧反应才能达到产物选择性的增加。虽然在我们的体系中产生了少量的OH自由基,它可以产生多个烷基羧酸自由基,但它们通过增加溶剂中乙腈的比例对整体产物的贡献非常有限。本文章由计算机程序翻译,如有差异,请以英文原文为准。
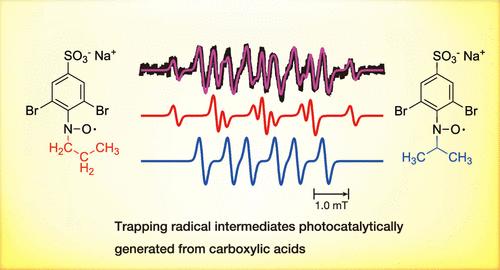
Radical Intermediates in Photocatalytic Activation of Carboxylic Acids: A Spin Trapping Study
Radicals generated by the photocatalytic reaction of carboxylic acids with a platinum-loaded TiO2 photocatalyst were trapped by a DBNBS (3,5-dibromo-4-nitrosobenzenesulfonate sodium salt) spin trapping agent, and the radical adducts were observed using electron spin resonance spectroscopy. In the case of ethanoic acid and propanoic acid in mixed solvents of water and acetonitrile, only adducts of methyl and ethyl radicals were observed as a result of decarboxylation reactions. The longer the alkyl chain of the linear alkyl carboxylic acid, the greater the percentage of adducts of secondary radicals and the higher the concentration of the total adduct. The results show that as the alkyl chain length increases, the diffusion rate of the generated radicals slows down. Additionally, during diffusion, intramolecular hydrogen transfer reactions take place. These reactions occur at a rate higher than that of the reaction with the spin-trapping reagent. However, not only the adducts of undecyl radicals but also those of dodecanoic acid radicals were detected by electrospray ionization mass spectrometry (ESI-MS), indicating that competitive reactions of radical cations ([HRCOOH]•+) of longer alkyl carboxylic acids to decarboxylation to produce an alkyl radical (HR•) or proton elimination to produce an alkyl carboxylic acid radical (•RCOOH) occur due to stabilization of radical cations by hyperconjugation with longer alkyl chain and the presence of water in the solvent as a proton acceptor. The same is true for branched alkylcarboxylic acids with tertiary carbon, which stabilizes the radical cations. Although this result also implies an increase in product diversity due to coupling between product radicals and a lack of product selectivity, we found that only the decarboxylation reaction proceeds when the percentage of water in the solvent is reduced to less than 5% to reach an increase in the product selectivity. Although OH radicals, which can produce multiple alkyl carboxylic acid radicals, were produced to a small extent in our systems, they made a very limited contribution to the overall product by increasing the ratio of acetonitrile in the solvent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


