化学选择性稳定的三苯基磷探针用于捕获活性羰基物种和丙烯酰胺弹头再生共价抑制剂
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
活性羰基物质(Reactive carbonyl species, RCS)具有高度亲电性,是氧化应激相关疾病的重要生物标志物。尽管它们有可能作为前药激活的触发因素,但RCS的选择性标记方法仍然有限。在这里,我们利用三苯基磷基团通过水溶液Wittig反应化学选择性捕获RCS,形成α,β-不饱和羰基,使其能够进一步功能化。我们首先设计了原生(轻)和氘化(重)探针,通过不同的MS同位素模式来促进RCS代谢组学鉴定。这种方法使我们能够捕获并相对量化与晚期脂氧化/糖基化终产物(ALEs/AGEs)相关的几种内源性RCS。其次,我们证明了各种内源性RCS可以触发具有不同取代基的靶向共价抑制剂(tci)的丙烯酰胺弹头的原位生成。这些结构变化影响了它们的蛋白质结合谱,从而改变了它们的细胞毒性,这有利于抑制剂鸡尾酒的开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
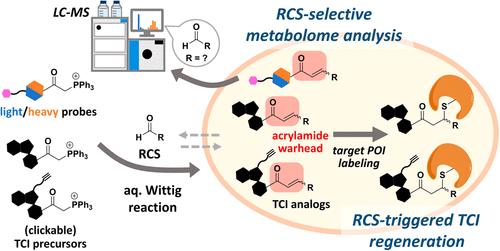
Chemoselective Stabilized Triphenylphosphonium Probes for Capturing Reactive Carbonyl Species and Regenerating Covalent Inhibitors with Acrylamide Warheads in Cellulo
Reactive carbonyl species (RCS) are important biomarkers of oxidative stress-related diseases because of their highly reactive electrophilic nature. Despite their potential as triggers for prodrug activation, selective labeling approaches for RCS remain limited. Here, we utilized triphenylphosphonium groups to chemoselectively capture RCS via an aqueous Wittig reaction, forming α,β-unsaturated carbonyls that enable further functionalization. We first designed native (light) and deuterated (heavy) probes to facilitate RCS metabolomic identification through distinct MS isotope patterns. This approach allowed us to capture and relatively quantify several endogenous RCS related to advanced lipoxidation/glycation end products (ALEs/AGEs). Second, we demonstrated that various endogenous RCS can trigger the in situ generation of acrylamide warheads of targeted covalent inhibitors (TCIs) with different substituents. These structural variations influence their protein binding profiles and consequently alter their cytotoxicity, which is beneficial for the development of inhibitor cocktails.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


