呋喃-巯基胺反应促进了dna相容性噻吩吡咯接枝的大环化和后期胺转化
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们在这里报告了一个高效的dna兼容呋喃-巯基胺反应,用于大环化和后期胺转化。该反应在温和的条件下进行,使dna偶联的线性肽能够很容易地环化成噻吩吡咯接枝的大环,而不考虑环的大小或侧链的修饰,转化率很高。此外,该策略还用于末端胺的后期转化,末端胺是构建dna编码肽库的关键中间体。不同的胺被成功地转化为相应的硫代吡咯支架,从而扩大了dna编码文库中可以实现的结构多样性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
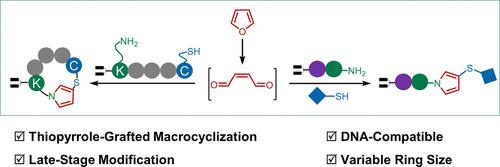
The Furan–Thiol–Amine Reaction Facilitates DNA-Compatible Thiopyrrole-Grafted Macrocyclization and Late-Stage Amine Transformation
We here report an efficient DNA-compatible furan–thiol–amine reaction for macrocyclization and late-stage amine transformation. This reaction, conducted under mild conditions, enables the facile cyclization of DNA-conjugated linear peptides into thiopyrrole-grafted macrocycles regardless of ring size or side-chain modification with good to excellent conversion yields. Additionally, this strategy was employed for the late-stage transformation of terminal amines, serving as critical intermediates in the construction of DNA-encoded peptide libraries. Diverse amines were successfully converted into their corresponding thiopyrrole scaffolds, thereby expanding the structural diversity that can be achieved within DNA-encoded libraries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


