核孔渗透率和流体流动受核孔膨胀状态的调节
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
不断变化的环境条件需要细胞质和核体积的快速适应。我们使用黏菌盘状钢(Dictyostelium disideum)来评估核孔复合物(npc)在实现核体积适应和缓解机械应力方面的作用。盘状钢以其耐受渗透压极端变化的能力而闻名。我们利用D. discoideum的独特特性来量化npc之间的流体流动。盘状蝶在原位有一个精细的鼻咽癌结构。其扩张状态影响鼻咽癌对核胞浆流动的渗透性。基于流体力学的数学概念,我们将这种现象概念化为npc之间的多孔流动,这与典型的核细胞质运输模式不同,因为它依赖于压力。病毒性鼻咽癌阻断减少核胞浆流量。我们的结果可能与任何需要快速核大小适应的生物学条件相关,包括转移癌细胞,迁移细胞或分化组织。本文章由计算机程序翻译,如有差异,请以英文原文为准。
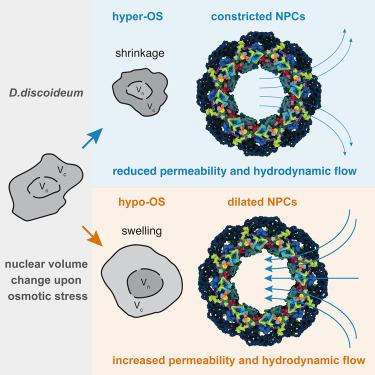
Nuclear pore permeability and fluid flow are modulated by its dilation state
Changing environmental conditions necessitate rapid adaptation of cytoplasmic and nuclear volumes. We use the slime mold Dictyostelium discoideum, known for its ability to tolerate extreme changes in osmolarity, to assess which role nuclear pore complexes (NPCs) play in achieving nuclear volume adaptation and relieving mechanical stress. We capitalize on the unique properties of D. discoideum to quantify fluid flow across NPCs. D. discoideum has an elaborate NPC structure in situ. Its dilation state affects NPC permeability for nucleocytosolic flow. Based on mathematical concepts adapted from hydrodynamics, we conceptualize this phenomenon as porous flow across NPCs, which is distinct from canonically characterized modes of nucleocytoplasmic transport because of its dependence on pressure. Viral NPC blockage decreased nucleocytosolic flow. Our results may be relevant for any biological conditions that entail rapid nuclear size adaptation, including metastasizing cancer cells, migrating cells, or differentiating tissues.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


