LDB1建立多增强子网络调控基因表达
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
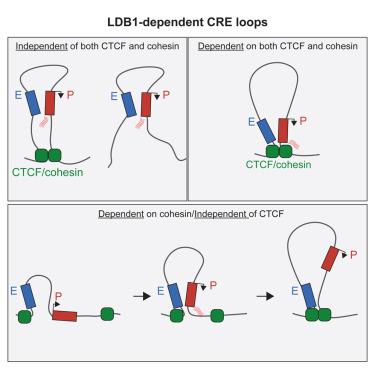
具体的增强子-启动子配对是如何建立的仍不清楚。除了CTCF/内聚蛋白机制外,很少有核因子被研究直接作用于物理连接调节元件。使用小鼠红细胞模型,我们通过急性降解实验表明,LDB1直接和广泛地促进调控元件之间的连通性。大多数ldb1介导的接触,即使是跨越数百kb的接触,也可以在缺乏CTCF、内聚蛋白或YY1的情况下形成,这是使用多degron系统确定的。此外,ldb1驱动的染色质环与内聚蛋白无关。内聚蛋白驱动的环挤压不会在ldb1占据的位点停止,而是有助于ldb1锚定环子集的形成。利用从有丝分裂到G1期过渡过程中核结构的动态重组,我们观察到环路形成和新生LDB1占用相关,并且可以独立于结构环路发生。Tri-C和区域捕获Micro-C表明LDB1组织多增强子网络来激活转录。这些发现表明LDB1是空间连通性的驱动因素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
LDB1 establishes multi-enhancer networks to regulate gene expression
How specific enhancer-promoter pairing is established remains mostly unclear. Besides the CTCF/cohesin machinery, few nuclear factors have been studied for a direct role in physically connecting regulatory elements. Using a murine erythroid cell model, we show via acute degradation experiments that LDB1 directly and broadly promotes connectivity among regulatory elements. Most LDB1-mediated contacts, even those spanning hundreds of kb, can form in the absence of CTCF, cohesin, or YY1 as determined using multiple degron systems. Moreover, an engineered LDB1-driven chromatin loop is cohesin independent. Cohesin-driven loop extrusion does not stall at LDB1-occupied sites but aids the formation of a subset of LDB1-anchored loops. Leveraging the dynamic reorganization of nuclear architecture during the transition from mitosis to G1 phase, we observe that loop formation and de novo LDB1 occupancy correlate and can occur independently of structural loops. Tri-C and Region Capture Micro-C reveal that LDB1 organizes multi-enhancer networks to activate transcription. These findings establish LDB1 as a driver of spatial connectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


