以1:1底物比选择性镍催化杂芳基氯化物和芳基溴化物的交叉亲电偶联
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
镍催化的(杂)芳基亲电试剂的交叉亲电偶联(XEC)反应是钯催化合成联芳基的有吸引力的替代方法,但它们通常会产生大量的均偶联和/或原脱卤副产物。在本研究中,利用杂芳酰氯和芳基溴偶联伙伴的信息库来确定当使用等摩尔量的两种底物时,镍催化的XEC条件对交叉产物具有高选择性。确定了两种不同的催化剂体系,它们具有互补的范围和广泛的官能团耐受性,并且时间过程数据表明这两种方法遵循不同的机制。以Zn为还原剂的NiBr2/三联吡啶催化体系将芳基溴转化为芳基锌中间体,与2-氯吡啶进行原位偶联;以四(二甲胺)乙烯为还原剂的NiBr2/联吡啶催化体系使用二硫化物和NaI作为添加剂实现选择性交叉偶联。本文章由计算机程序翻译,如有差异,请以英文原文为准。
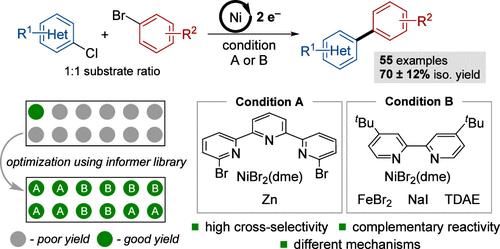
Selective Ni-Catalyzed Cross-Electrophile Coupling of Heteroaryl Chlorides and Aryl Bromides at 1:1 Substrate Ratio
Nickel-catalyzed cross-electrophile coupling (XEC) reactions of (hetero)aryl electrophiles represent appealing alternatives to palladium-catalyzed methods for biaryl synthesis, but they often generate significant quantities of homocoupling and/or proto-dehalogenation side products. In this study, an informer library of heteroaryl chloride and aryl bromide coupling partners is used to identify Ni-catalyzed XEC conditions that access high selectivity for the cross-product when using equimolar quantities of the two substrates. Two different catalyst systems are identified that show complementary scope and broad functional-group tolerance, and time-course data suggest that the two methods follow different mechanisms. A NiBr2/terpyridine catalyst system with Zn as the reductant converts the aryl bromide into an arylzinc intermediate that undergoes in situ coupling with 2-chloropyridines, while a NiBr2/bipyridine catalyst system with tetrakis(dimethylamino)ethylene as the reductant uses FeBr2 and NaI as additives to achieve selective cross-coupling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


