序贯钯催化Aza-Heck/Suzuki偶联反应对映选择性合成异吲哚酮
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们提出了一个串联aza-Heck/Suzuki交叉偶联反应,邻苯羟肟醚与容易获得的芳硼酸和烯基硼酸。该方案是通过钯催化剂与手性磷酰胺配体配对,特别是在温和的反应条件下,产生高效和简洁的手性异吲哚酮的合成路线,具有高对映选择性。此外,该反应具有良好的官能团相容性和丰富的后续转化多样性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
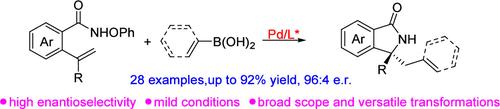
Enantioselective Synthesis of Isoindolinone by Sequential Palladium-Catalyzed Aza-Heck/Suzuki Coupling Reaction
We present a tandem aza-Heck/Suzuki cross-coupling reaction of O-phenyl hydroxamic ethers with readily available arylboronic and alkenyl boronic acids. This protocol is enabled by a palladium catalyst paired with chiral phosphoramidite ligands, particularly under mild reaction conditions, yielding efficient and succinct synthetic routes to chiral isoindolinones with high enantioselectivity. Furthermore, this reaction exhibits excellent functional group compatibility and a rich diversity of subsequent transformations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


